Consider only the species (at standard conditions) [mathrm{Na}^{+}, mathrm{Cl}^{-}, mathrm{Ag}^{+}, mathrm{Ag}, mathrm{Zn}^{2+}, mathrm{Zn} text {, and }
Question:
Consider only the species (at standard conditions)
\[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\]
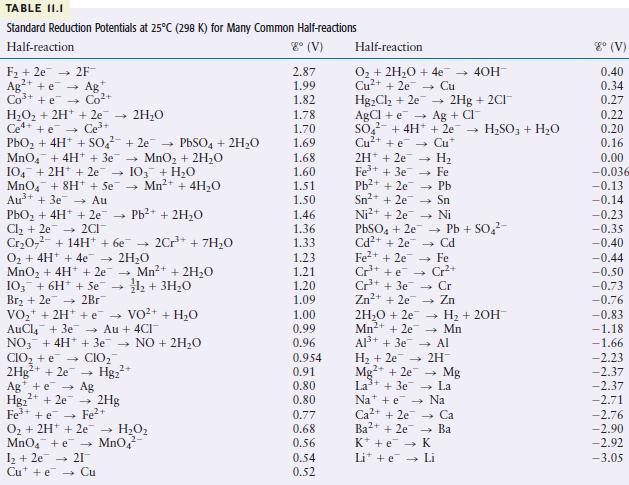
in answering the following questions. Give reasons for your answers. (Use data from Table 11.1.)
a. Which is the strongest oxidizing agent?
b. Which is the strongest reducing agent?
c. Which species can be oxidized by \(\mathrm{SO}_{4}{ }^{2-}(a q)\) in acid?
d. Which species can be reduced by \(\mathrm{Al}(s)\) ?
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7H0 O + 4H+ + 4e 2HO MnO + 4H+ + 2e 4 Mn+ + 2HO 1+ 3HO 103 + 6H + Se Br +2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ Ag + e 2+ Hg+ + 2e Fe+ + e O + 2H+ 2e MnO4 + e 1 +2e 21 Cute Cu Ag 2Hg Fe+ HO MnO4 + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO + 4H+ Cu+ + e 2H+2e7 4 H 1 Fe+ + 3e7 Pb+ + 2e7 Fe Pb - Sn Ni Sn+ + 2e Ni+ + 2e PbSO4 + 2e7 Cd+ + 2e Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e- HSO3 + HO Cu* Cd - Fe Cr+ Cr Zn - H + 2OH- Mn Al Pb + SO - 40H- H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e7 Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To answer these questions we will refer to the reduction potentials listed in the provided table Standard reduction potentials indicate the tendency o...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the relation shown in Figure 30.2(d). How would it appear to a user with classification U? Suppose that a classification U user tries to update the salary of Smith to $50,000; what would be...
-
On October 10, the stockholders' equity section of Sherman Systems appears as follows. Common stock-$10 par value, 5,050 shares authorized, issued, and outstanding Paid-in capital in excess of par...
-
Consider only the species (at standard conditions) Br 2 , Br 2 , H + , H 2 , La 3+ , Ca, Cd in answering the following questions. Give reasons for your answers. a. Which is the strongest oxidizing...
-
Suppose there are two identical forest plots except that one will be harvested and left as is while the second will be cleared after the harvest and turned into a housing development. In terms of...
-
A gun fires a projectile of mass 10kg of the type to which the curves of Figure 2-3 apply. The muzzle velocity is 140m/s. Through what angle must the barrel be elevated to hit a target on the same...
-
Urban Shocker has noted that the spread between the yield-to-maturity on BBB-rated bonds and that on AAA-rated bonds has recently widened considerably. Explain to Urban what this change might...
-
Read the cases studies of Oticon (Chapter 10 Case), W.L. Gore (Part 5 Case) and Pixar (pages 309 and 403), and note any similarities and differences between their practices and those of Google.
-
Dana Corporation, based in Toledo, Ohio, is a global manufacturer of highly engineered products that serve industrial, vehicle, construction, commercial, aerospace, and semiconductor markets. It...
-
9-5A Comprehensive Variance Analysis Problem Chemco produces chemicals for cleaning pools. It sells the chemicals (a powder) in four kilogram buckets. The company's standard costs per unit follow:...
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution). a. oxidizes...
-
The saturated calomel electrode, abbreviated SCE, is often used as a reference electrode in making electrochemical measurements. The SCE is composed of mercury in contact with a saturated solution of...
-
Which of the following declares a Comparator where all objects are treated as equal? A. Comparator comp = (c1) -> 0; B. Comparator comp = (c1) -> {0}; C. Comparator comp = (c1, c2) -> 0; D....
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
Starting with Eq. 10-20, show that the exergy destruction associated with a simple ideal Rankine cycle can be expressed as xdest = qin(th,Carnot - th), where th is efficiency of the Rankine cycle and...
-
A fast-food restaurant averages 150 customers per hour. The average processing time per customer is 90 seconds. a. Determine how many cash registers the restaurant should have if it wishes to...
-
Calculate the pH of 6.55 * 10 7 m HClO 4 (aq).
-
One of the largest uses of electricity is in the production of aluminum by electrolysis of its oxide dissolved in molten cryolite (Na 3 AlF 6 ). As an engineer, you might need to predict how much...
-
You are working in an analytical laboratory and have been asked to use the permanganate solution you prepared in Example 6K.1 to determine the concentration of bromide ions in a sample of groundwater...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...

Study smarter with the SolutionInn App


