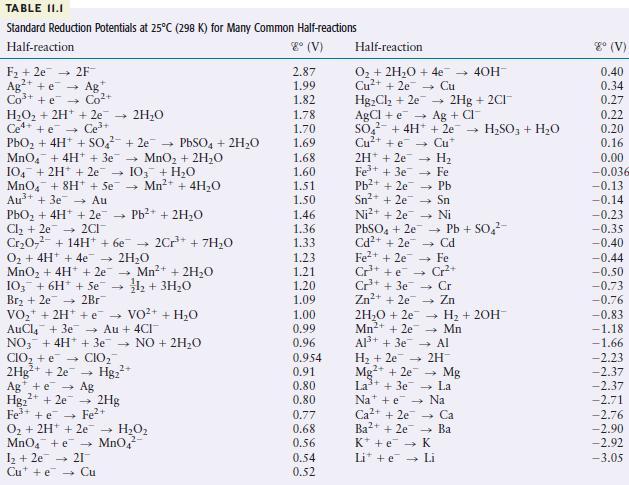
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of
Question:
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution).
a. oxidizes \(\mathrm{Br}^{-}\)to \(\mathrm{Br}_{2}\) but does not oxidize \(\mathrm{Cl}^{-}\)to \(\mathrm{Cl}_{2}\)
b. oxidizes \(\mathrm{Mn}\) to \(\mathrm{Mn}^{2+}\) but does not oxidize \(\mathrm{Ni}\) to \(\mathrm{Ni}^{2+}\)
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7H0 O + 4H+ + 4e 2HO MnO + 4H+ + 2e 4 Mn+ + 2HO 1+ 3HO 103 + 6H + Se Br +2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ Ag + e 2+ Hg+ + 2e Fe+ + e O + 2H+ 2e MnO4 + e 1 +2e 21 Cute Cu Ag 2Hg Fe+ HO MnO4 + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO + 4H+ Cu+ + e 2H+2e7 4 H 1 Fe+ + 3e7 Pb+ + 2e7 Fe Pb - Sn Ni Sn+ + 2e Ni+ + 2e PbSO4 + 2e7 Cd+ + 2e Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e- HSO3 + HO Cu* Cd - Fe Cr+ Cr Zn - H + 2OH- Mn Al Pb + SO - 40H- H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e7 Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
We must search for reagents with reduction potentials larger than the reduction potentials of the s...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following reductions (under standard conditions in acidic solution). a. reduces...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2 and extracting the Br 2 from the solution using an organic solvent. Write a balanced...
-
In Table 12. 1, when r = 0. 02, the present value of the cost rises for 68 years and then subsequently declines. Why? Table 12. 1 TABLE 12.1 Economic Harvesting Decision: Douglas Fir 10 20 30 40 50...
-
Show directly that the time rate of change of the angular momentum about the origin for a projectile fired from the origin constant g is equal to the moment force or torque about the origin.
-
Corporate default seems to be an event specific to an individual company. Yet despite the apparent diversifiable nature of corporate default (meaning that relatively few bonds would default in a...
-
Identify for an employee (perhaps yourself) what knowledge he/she creates, acquires, captures, shares and uses while doing a specified task.
-
You are an audit supervisor assigned to a new client, Go-Go Corporation, which is listed on the New York Stock Exchange. You visited Go-Gos corporate headquarters to become acquainted with key...
-
Case Study Crush Ltd. is a dealer in fast-moving consumer goods. The Company has warehouses throughout the country where the stocks are stored. The Auditor of the Company normally conducts physical...
-
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing \(\mathrm{Mn}^{2+}\) ions. These ions are then oxidized to...
-
Consider only the species (at standard conditions) \[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\] in answering the...
-
Multi-National Enterprises (MNE) operates in two countries, X and Y, with tax rates of 40 percent and 10 percent respectively. Production costs are exactly the same in each country. The following...
-
See US Debt Clock and answer the following: (Hint: Take a screenshot of the Debt Clock) (2) A. What is the current US deficit and the total federal debt? (1) B What is the net interest...
-
GASB states that public colleges and universities are special purpose governmentsand therefore accountable to the citizenry (Hoyle, 2015). Furthermore, GASB found that for public colleges and...
-
You have recently been assigned to the production planning department within your company. Your firm makes large blades for power generation windmills. The windmills are mostly used in the western...
-
Jason Ready attended the University of Ohio from 2 0 1 9 to 2 0 2 3 under the Air Force ROTC program. Excluding the school expenses covered by his ROTC scholarship, he incurred additional school...
-
Questions Chap 1 1. Consider the following cases and decide whether criminal or civil proceedings would result, and make a note of the parties in the action. a) Ali is being prosecuted for careless...
-
A steam power plant operates on a simple ideal Rankine cycle between the pressure limits of 3 MPa and 50 kPa. The temperature of the steam at the turbine inlet is 300C, and the mass flow rate of...
-
A sprinkler head malfunctions at midfield in an NFL football field. The puddle of water forms a circular pattern around the sprinkler head with a radius in yards that grows as a function of time, in...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A 1.0 m KBr(aq) solution...
-
(a) What concentration (in moles per liter) of Ag + ions is required for the formation of a precipitate in 1.0 * 10 5 m NaCl(aq)? (b) What mass (in micrograms) of solid AgNO 3 needs to be added for...
-
Although molar solubilities are often of interest, you might find it difficult to locate the appropriate data. Solubility constants, however, are often easier to find, and can be converted to molar...
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


