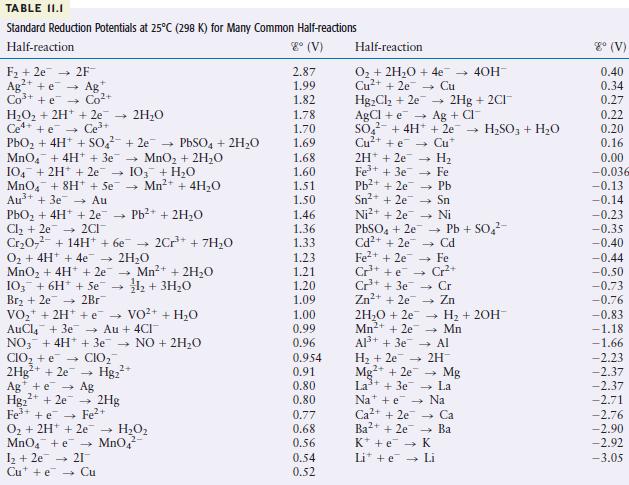
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of
Question:
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following reductions (under standard conditions in acidic solution).
a. reduces \(\mathrm{Cu}^{2+}\) to \(\mathrm{Cu}\) but does not reduce \(\mathrm{Cu}^{2+}\) to \(\mathrm{Cu}^{+}\)
b. reduces \(\mathrm{Br}_{2}\) to \(\mathrm{Br}^{-}\)but does not reduce \(\mathrm{I}_{2}\) to \(\mathrm{I}^{-}\)
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7HO O + 4H+ + 4e 2HO MnO + 4H+ + 2e 103 + 6H + Se Br + 2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ - 4 Mn+ + 2HO 1+ 3HO Ag + e Ag 2+ Hg+ + 2e 2Hg Fe+ + e Fe+ O + 2H+ 2e HO MnO4 + e MnO4 1 +2e 21 Cute Cu + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO4 + 4H+ Cu+ +e 2H+2e7 4 1 Fe+ + 3e7 Pb+ + 2e7 Sn+ + 2e Ni+ + 2e H Fe Pb PbSO4 + 2e7 Cd+ + 2e 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e HSO3 + HO Cu* - Sn Ni Pb + SO Cd - Fe Cr+ Cr Zn Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 - - 40H- H + 2OH- Mn Al H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To answer these questions we need to look at the table of standard reduction potentials and select reagents that can reduce the specified species with...View the full answer

Answered By

Sufiyan Ahmed Tariq
I am a Chartered Accountant and an Associate Public & Finance Accountant. I also hold a bachelors of Commerce degree. I have over 8 years of experience in accounting, finance and auditing. Through out my career, I have worked with many leading multinational organisation.
I have helped a number of students in studies by teaching them key concepts of subjects like accounting, finance, corporate law and auditing. I help students understanding the complex situation by providing them daily life examples.
I can help you in the following subject / areas:
a) Accounting;
b) Finance;
c) Commerce;
d) Auditing; and
e) Corporate Law.
4.90+
7+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution). a. oxidizes...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2 and extracting the Br 2 from the solution using an organic solvent. Write a balanced...
-
As our energy structure transitions toward renewable fuels, forest-based biomass fuels benefit from this transition. What are the likely effects of this transition on consumers, producers, and the...
-
The motion of a charged particle in an electromagnetic field can be obtained from the Lorentz equation for the force on a particle in such a field, if the electric field vector is E and the magnetic...
-
Camp Douglas Dirigibles has a bond outstanding with four years to maturity, a face value of $1,000, and an annual coupon payment of $100. What is the price of the Camp Douglas bond if its...
-
Identify examples of explicit and tacit knowledge in this example.
-
A sample of 10 NCAA college basketball game scores provided the following data. a. Compute the mean and standard deviation for the points scored by the winning team. b. Assume that the points scored...
-
Saxe Banners reported the following figures in its financial statements: Cash 29,000 29,500 Cash Equivalents Total Current Liabilities 45,000 Compute the cash ratio for Saxe Banners. - Determine the...
-
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing \(\mathrm{Mn}^{2+}\) ions. These ions are then oxidized to...
-
Consider only the species (at standard conditions) \[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\] in answering the...
-
Set up, but do not evaluate, an integral for the volume of the solid obtained by rotating the region bounded by the given curves about the specified axis. y = cos2x, |x| /2, y = 1/4; about x = /2
-
1. Mainland purchased a machine for $85,000 on 1 January 20x7 and assigned it a useful life for 10 years. On 31 March 20x9 it was revalued to $93,000 with no change in useful life. Complete the table...
-
Find the equation of the regression line and identify a characteristic of the data that is ignored by the regression line X 10 8 13 9 11 14 6 4 12 7 5 Y 7.46 6.77 12.74 7.11 7.81 8.84 6.08 5.39 8.15...
-
For each of the following independent cases, fill in the missing amounts in the table: (Indicate the effect of each variance by selecting "F" for favorable, "U" for unfavorable.) Case Direct Labor...
-
All views expressed in this paper are those of the authors and do not necessarily represent the views of the Hellenic Observatory or the LSE George Alogoskoufis Greeces Sovereign Debt Crisis:...
-
Current Attempt in Progress Nash Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,812,000 on March 1, $1,212,000 on June 1,...
-
Refrigerant-134a is used as the working fluid in a simple ideal Rankine cycle which operates the boiler at 2000 kPa and the condenser at 24C. The mixture at the exit of the turbine has a quality of...
-
Repeat the previous problem, but close the positions on September 20. Use the spreadsheet to find the profits for the possible stock prices on September 20. Generate a graph and use it to identify...
-
Determine Ksp for each of the following sparingly soluble compounds, given their molar solubilities: (a) AgI, 9.1 * 1029 mol L 1 ; (b) Ca(OH) 2 , 0.011 mol L 1 ; (c) Ag 3 PO 4 , 2.7 * 1026 mol L 1...
-
The concentration of mercury, a toxic heavy metal pollutant, in aqueous solution depends in part on the redox properties of its compounds. Suppose you are studying the properties of mercury. You...
-
The following redox reaction is used in acidic solution to prepare orthotelluric acid: Identify the elements undergoing oxidation or reduction and indicate their initial and final oxidation numbers....
-
Suppose you bought a bon with an annual coupon rate of 6.5 percent one year ago for $1,032. The bond sells for $1,020 today. a. Assuming a $1,000 face value, what was your total dollar return on this...
-
During the year 2021, William has a job as an accountant, he earns a salary of $100,000. He has done some cleaning services work on his own (self-employed), where he earned a net income of $50,000....
-
Fixed cost per unit is $7 when 25,000 units are produced and $5 when 35,000 units are produced. What is the total fixed cost when 30,000 units are produced? Group of answer choices $150,000....

Study smarter with the SolutionInn App


