K sp for Cu(IO 3 ) 2 is 1.4 * 10 7 . Using this value and
Question:
Ksp for Cu(IO3)2 is 1.4 * 10–7. Using this value and data in Appendix 2B, calculate E° for the half-reaction Cu(IO3)2(s) + 2 e– → Cu(s) + 2 IO3 –(aq).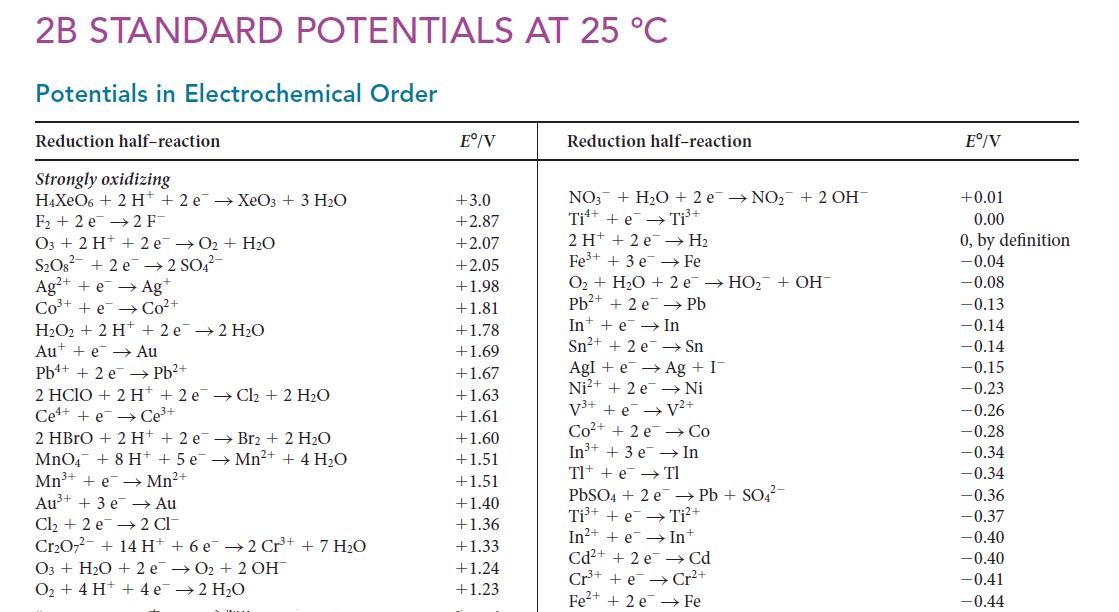
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6+ 2 H+2 e → XeO3 + 3 H₂O F₂2 e 2 F- O3 + 2 H+ 2 e → O₂ + H₂O S₂O8² +2e → 2 SO4²- Ag²+ +e → Agt Co³ + e Co²+ H₂O2+ 2 H+ 2e → 2 H₂O Aue Au Pb²+ Pb+ + 2 e 2 HCIO + 2 H+2 e → Cl₂ + 2 H₂O Cee Ce³+ 2 HBrO + 2 H+2 e Br2 + 2 H₂O MnO4 + 8 H+ + 5 e →Mn²+ + 4H₂O Mn³+ + e→ Mn²+ Au³+ + 3 e →→ Au Cl₂ + 2 e 2 CI Cr₂O7² + 14 H+ + 6 e2 Cr³+ + 7 H₂O O3 + H₂O + 2e →O₂ + 2 OH →2 H₂O O₂ + 4 H+ + 4e Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + H₂O + 2 e → NO₂+ 2 OH Ti + e Ti³+ → H₂ Fe O₂ + H₂O + 2 e → HO₂ + OH Pb²+ + 2 e Pb In e Sn²+ + 2 e Sn AgIe → Ag + I Ni²+ + 2e → Ni V³+ + e → V²+ Co²+ +2e In³+ + 3 e Tl + e 2H+ +2 e Fe³+ + 3 e In → Co In Tl PbSO4 + 2 e Pb + SO4²- Ti³+ + e Ti²+ In²++eIn+ Cd²+ + 2 e Cd Cr³+e Cr²+ Fe²+ + 2 e Fe Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
K sp for Ni(OH) 2 is 6.5 * 10 18 . Use this value and data from Appendix 2B to calculate E for the half-reaction Ni(OH) 2 (s) + 2 e Ni(s) + 2 OH (aq). 2B STANDARD POTENTIALS AT 25 C Potentials in...
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
An investor creates an investment portfolio from stock A and stock B where she invests 0.5 of her wealth in stock A and 0.5 of her wealth in stock B. Stock A has a beta of 1.39 and stock B has a beta...
-
Multinational transfer pricing, global tax minimization. Industrial Diamonds, Inc., based in Los Angeles, has two divisions: South African Mining Division, which mines a rich diamond vein in South...
-
In Figure a metal rod is forced to move with constant velocity v along two parallel metal rails, connected with a strip of metal at one end. A magnetic field of magnitude B = 0.350 T points out of...
-
Describe how software can assist in project scope management? LO.1
-
Using the data presented in E1-13, determine the amount Fortune Corporation would record as a gain on bargain purchase and prepare the journal entry Fortune would record at the time of the exchange...
-
Perpetual Inventory Using UFO Beginning inventory, purchases, and sales data for DVD players are as follows: November 1 Inventory 61 units at $62 10 Sale 48 units 15 Purchase 77 units at $65 20 Sale...
-
Effective financial statement analysis requires an understanding of a firms economic characteristics. The relations between various financial statement items provide evidence of many of these...
-
A galvanic cell has the following cell reaction: M(s) + 2 Zn 2+ (aq) 2 Zn(s) + M 4+ (aq). The standard potential of the cell is 10.16 V. What is the standard potential of the M 4+ /M redox couple?
-
Arrange the following metals in order of increasing strength as reducing agents: U, V, Ti, Ni, Sn, Cr, Rb.
-
On January 12, 2013, Mr. and Mrs. Nixon moved out of their old residence (where they had lived for 22 years) and into a new residence purchased nine days earlier on January 3. They nally sold their...
-
Could I obtain assistance with these . problems? 1. Find the coordinates of the turning points of the curve y=3x^4-8x^3-30x^2+72x+5. Determine the nature of these points. "Determine the nature"...
-
1 . In 1 9 6 0 the homeownership rate in the United States was 6 2 % . Is there evidence to indicate that the homeownership rate is now higher? To answer the question, the researchers sample 5 0 2...
-
A certain disease is classified into 4 stages that distinguish how developed the disease is. Researchers studying a new potential treatment recruited over 100 patients with varying stages of the...
-
1. (20) Let and Dor {abnm or 2n m} = Dand = {a"b" nm and 2n m}. Prove that Dor and Dand are both context-free.
-
Given n samples 1 , 2 , . . . , x 1 ,x 2 ,...,x N drawn independently from a Poisson distribution unknown parameter , find the MLE of . = = 1 MLE = i=1 n x i = = 1 MLE =n i=1 n x i = = 1 MLE = i=1 n...
-
The following table shows the number of bacteria colonies present in each of several petri dishes, after E. coli bacteria were added to the dishes and they were incubated for 24 hours. The "soap"...
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
When benzene is treated with D 2 SO 4 , a deuterium atom replaces one of the hydrogen atoms. Propose a mechanism for this reaction. Once again, make sure that your mechanism involves a sigma complex....
-
Calculate KP at 298 and 490. K for the reaction NO(g) + 1/2O 2 (g) NO 2 (g) assuming that H o R is constant over the interval 298600. K. Do you expect K p to increase or decrease as the temperature...
-
Draw the mechanism of the following reaction, and make sure to draw all three resonance structures of the sigma complex. NO, HNO3 H,SO,
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


