Suppose that 0.483 g of an unknown weak acid, HA, is dissolved in water. Titration of the
Question:
Suppose that 0.483 g of an unknown weak acid, HA, is dissolved in water. Titration of the solution with 0.250 m NaOH(aq) required 42.0 mL to reach the stoichiometric point. After the addition of 21.0 mL, the pH of the solution was found to be 3.75.
(a) What is the molar mass of the acid?
(b) What is the value of pKa for the acid? Identify the acid in Table 6C.1.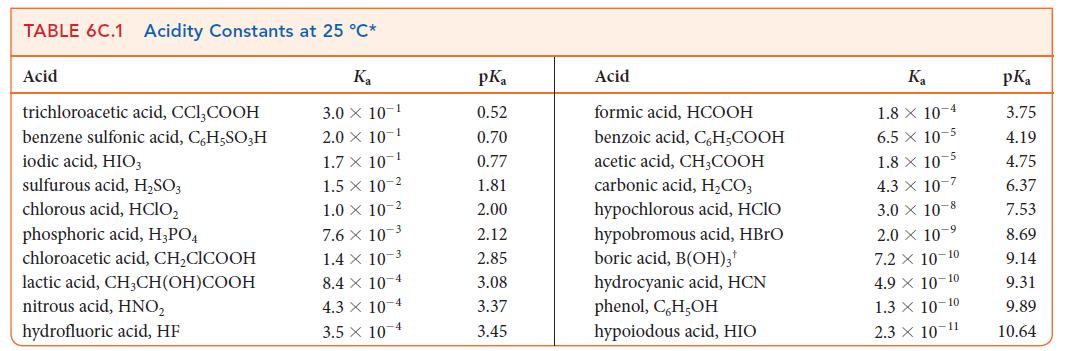
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* Ka 3.0 X 10-1 2.0 X 10-1 1.7 X 10-¹ Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H,SO3 H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂ClCOOH lactic acid, CH3CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF 1.5 x 10-2 1.0 10-2 7.6 x 10-3 1.4 x 10-3 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK₁ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C6H₂COOH acetic acid, CH,COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K₁ 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 x 10-7 3.0 10-8 2.0 × 10-⁹ 7.2 x 10-10 4.9 × 10-10 1.3 × 10-10 2.3 × 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Lets start by calculating the molar mass of the acid a Given Mass of HA 0483 g Volume of NaOH 420 mL ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write a memo to explain to your manager how a parent should consolidate its subsidiarys financial statement. Introduction (Paragraph One) This section describes the purpose of the memo or reminds...
-
1 = 47 lb/ ft 2 = 140 lb/ft h= 11.3 ft Best Score: 2.00 / 4 Current Score: 2.00 /4 The weight of the water. 1/1 pts v System Answer: 25384.32 The weight of the maple slab. 1/1 pts v System Answer:...
-
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.10 and 6H.12. Exercises 6H.10 Morphine, C 17 H 19 O 3 N, is a potent painkiller. Suppose you are studying...
-
Giroud plc is considering two alternative investment opportunities. Each of the two projects has an expected life of five years and requires an initial investment of $100,000. A feasibility study...
-
Joint-cost allocation with a byproduct. The Cumberland Mine is a small mine that extracts coal in West Virginia. Each ton of coal mined is 40% Grade A coal, 40% Grade B coal, and 20% coal tar. All...
-
Why do persistent programming languages allow transient objects? Might it be simpler to use only persistent objects, with unneeded objects deleted at the end of an execution? Explain your answer.
-
Bin the data into three bins of two records each.
-
Hill Propane Distributors wants to construct a pro forma balance sheet for 2013. Build the statement using the following data and assumptions: 1. Projected sales for 2013 are $35 million. 2. Hills...
-
The following information is available for Eliza Corporation for 2 0 2 3 : 1 . Depreciation reported on the tax return exceeded depreciation reported on the income statement by $ 2 0 1 , 0 0 0 . The...
-
Consider the 2013 declined loan data from LendingClub titled RejectStatsB2013. Similar to the analysis done in the chapter, lets scrub the employment length. Because our analysis requires risk...
-
Suppose that each of the following pairs of redox couples is combined to form a galvanic cell that generates a current under standard conditions. Identify the oxidizing agent and the reducing agent,...
-
Write the chemical equations of the two proton transfer equilibria that demonstrate the amphiprotic character of (a) H 2 PO 3 ; (b) NH 3 . Identify the conjugate acidbase pairs in each case.
-
The value V of an item t years after it is purchased is V = 9000e -0.6t for 0 t 5. (a) Use a graphing utility to graph the function. (b) Find the rates of change of V with respect to t when t = 1...
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
On January 1, Mitzu Company pays a lump-sum amount of $2,700,000 for land, Building 1, Building 2, and Land Improvements 1. Building 1 has no value and will be demolished. Building 2 will be an...
-
Invertase is an enzyme that may aid in spore germination of the fungus Colletotrichum graminicola. A botanist incubated specimens of the fungal tissue in petri dishes and then assayed the tissue for...
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
You have collected a tissue specimen that you would like to preserve by freeze drying. To ensure the integrity of the specimen, the temperature should not exceed 5.00C. The vapor pressure of ice at...
-
The phase diagram of NH 3 can be characterized by the following information. The normal melting and boiling temperatures are 195.2 and 239.82 K, respectively; the triple point pressure and...
-
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical method or a least squares fitting routine. T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230....
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


