Question: Use the data in the following table for three different aqueous solutions of (mathrm{CaCl}_{2}) to calculate the apparent value of the van't Hoff factor. Molality
Use the data in the following table for three different aqueous solutions of \(\mathrm{CaCl}_{2}\) to calculate the apparent value of the van't Hoff factor.
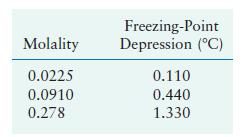
Molality 0.0225 0.0910 0.278 Freezing-Point Depression (C) 0.110 0.440 1.330
Step by Step Solution
3.36 Rating (146 Votes )
There are 3 Steps involved in it
To calculate the apparent value of the vant Hoff factor i we can use the colligative property of fre... View full answer

Get step-by-step solutions from verified subject matter experts


