Use the phase diagram for compound X below to answer these questions: (a) Is X a solid,
Question:
Use the phase diagram for compound X below to answer these questions:
(a) Is X a solid, liquid, or gas at normal room temperatures?
(b) What is the normal melting point of X?
(c) What is the vapor pressure of liquid X at 250°C?
(d) What is the vapor pressure of solid X at 2100°C?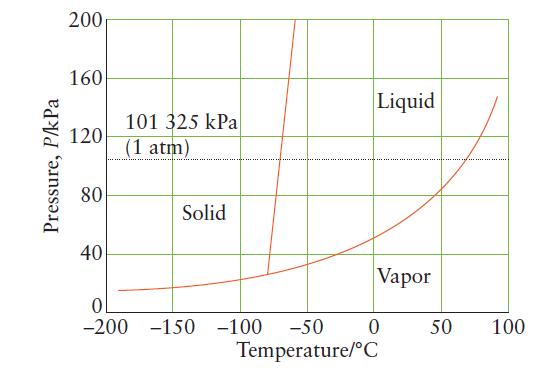
Transcribed Image Text:
Pressure, P/kPa 200 160 120 80 40 101 325 kPa (1 atm) 0 -200 Solid -150 -100 -50 Liquid Vapor 0 Temperature/C 50 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a X is a liquid at normal room temperature ...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The phase diagram for neon is Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will...
-
Specify the characteristics( r , r and ) of a material in which 100MHz uniform plane wave would have a wavelength of 1m, an attenuation of 2Np/m and an intrinsic impedance of 200 ohms.
-
A student wants to test whether a modification to a machine will improve its efficiency. The student takes ten measurements without the modification (case A), and ten measurements with the...
-
In Exercises 35 through 42, the slope f'(x) at each point (x, y) on a curve y = f(x) is given along with a particular point (a, b) on the curve. Use this information to find f(x). f'(x) = 3x 2 + 6x ...
-
The disproportionation of toluene to benzene and xylenes is carried out in a catalytic reactor at 500 psia and 950?F. The reactor effluent is cooled in a series of heat exchangers for heat recovery...
-
A sample of an ideal gas is in a vertical cylinder fitted with a piston. As 5.79 kJ of energy is transferred to the gas by heat to raise its temperature, the weight on the piston is adjusted so that...
-
Contrast accounting for pensions to accounting for other postretirement benefits.
-
In recent years, the financial press reported story after story regarding downsizing and layoff s in the U.S. auto industry. Often the stories discussed multiple rounds of downsizing events (such as...
-
Exercise 9-4 (Algo) Prepare a Flexible Budget Performance Report [LO9-4] Vulcan Flyovers offers scenlc overflights of Mount SaInt Helens, the volcano In WashIngton State that explosively erupted In...
-
When Serena was dismissed without cause from her position as vice president, she received only her entitlements under the Employment Standards Act, 2000. Her compensation package was variable so, as...
-
The density of 14.8 m NH 3 (aq) is 0.901 g cm 3 . What is the molality of NH 3 in the solution?
-
If Q = 1.0 for the reaction N 2 (g) + O 2 (g) 2 NO(g) at 25C, will the reaction have a tendency to form products or reactants, or will it be at equilibrium?
-
LO7 Art is in the 28% marginal tax bracket for 2010. He owes a $10,000 bill for business expenses. Because he reports taxable income on a cash basis, he can deduct the $10,000 in either 2010 or 2011,...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
(a) Describe a TCP session opening. (b) Describe a normal TCP closing. (c) Describe an abrupt TCP closing. (d) Describe how reliability is implemented in TCP. (e) Describe a TCP half-open DoS attack....
-
Sportique Boutique reported the following financial data for 2012 and 2011. Instructions(a) Calculate the current ratio for Sportique Boutique for 2012 and 2011.(b) Suppose that at the end of 2012,...
-
Draw a mechanism for the following intramolecular process:
-
The triphenylmethyl radical reacts with itself to form the following dimer: Identify the type of radical process taking place, and draw the appropriate fishhook arrows. 2.
-
Show that (C V /V) T = T( 2 P/ T 2 ) V
-
Use the following information: \ table [ [ Country , \ table [ [ Consumer Prices ] ] , Interest Rates,Current Units ( per US$ ) ] , [ Forecast , 3 - month, 1 - yx Covt Bond,, ] , [ 2 0 2 4 e ,...
-
Year-to-date, Yum Brands had earned a 3.70 percent return. During the same time period, Raytheon earned 4.58 percent and Coca-Cola earned 0.53 percent. If you have a portfolio made up of 40 percent...
-
Rate of Return If State Occurs State of Probability of Economy State of Economy Stock A Stock B Stock C Boom .15 .31 .41 .21 Good .60 .16 .12 .10 Poor .20 .03 .06 .04 Bust .05 .11 .16 .08 a. Your...

Study smarter with the SolutionInn App


