Using data in Appendix 2B, calculate the standard potential for the half-reaction Ti 4+ (aq) + 4
Question:
Using data in Appendix 2B, calculate the standard potential for the half-reaction Ti4+(aq) + 4 e– → Ti(s).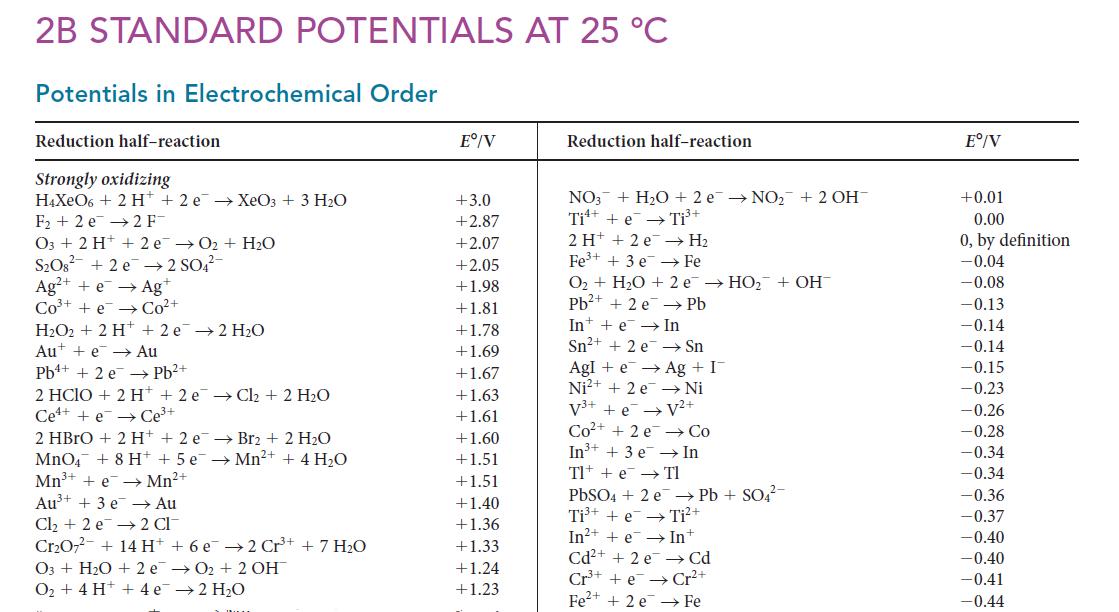
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F2 e 2 F- O3 + 2 H+ 2 e 0 + HO SO8 +2e 2 SO4- Ag+ + e Ag+ CO+ +e Co+ HO2 + 2 H + 2e 2 H0 Aue Au Pb+ Pb + 2 e 2 HCIO + 2 H+2 e Cl + 2 HO Cee Ce+ 2 HBrO + 2 H+ + 2 e Br2 + 2 HO MnO4 + 8 H+ + 5 e Mn+ + 4HO Mn+ + e Mn+ 2 CI Au+ + 3 e Au Cl + 2 e CrO7 + 14 H O3+ HO + 2e O + 4 H+ + 4e + 6 e2 Cr+ + 7 HO O + 2 OH 2HO E/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + HO + 2 e NO + 2 OH Ti + e Ti+ 2H+ +2 e Fe+ + 3 e H Fe O + HO + 2e HO + OH Pb+ + 2 e Pb In e In Sn+ + 2 e Sn AgI+eAg + I Ni2+ + 2e Ni V+ + e V+ Co+ +2e Co In+ + 3 e In Tl + e Tl PbSO4 + 2 e Pb + SO4- Ti+ + e Ti+ In++eIn+ Cd+ + 2 e Cd Cr+e Cr+ Fe+ + 2 e Fe E/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
The appropriate halfreactions Ti e Ti E 000 A Ti e Ti E 037 B Ti 2e Ti E 163 C A B and ...View the full answer

Answered By

KIRAN P
I have M TECH in Chemical Engineering from one of India's best Institute NIT TRICHY and have more than 10 years experience in Chemical industry.Used to teach students during leisure times
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e...
-
Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F+2 e...
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
Elizabeth Is a nurse, and she just administered 1.8 milliliters of medication to one of her patients. Elizabeth knows that the amount of medication remaining in the patient's body wi decrease by a...
-
Effect of alternative transfer-pricing methods on division operating income. Crango Products is a cranberry cooperative that operates two divisions: a Harvesting Division and a Processing Division....
-
A circular loop of wire 50 mm in radius carries a current of 100 A. Find the (i) Magnetic field strength and (ii) Energy density at the center of the loop.
-
Which tool or technique for collecting requirements is often the most expensive and time consuming? a. interviews b. focus groups c. surveys d. observation LO.1
-
In this mini-case you will audit and evaluate documents such as bank confirmations and bank reconciliations in the audit of one of EarthWear's cash accounts. The company has several other cash...
-
The Expected return on Portfolio A made of 35% , 46% and 19% of X, Y and Z respectively is
-
A polling company reported that 27% of 2302 surveyed adults said that they play baseball. Complete parts (a) through (d) below. a. What is the exact value that is 27% of 2302? The exact value is...
-
Arrange the following metals in order of increasing strength as reducing agents: U, V, Ti, Ni, Sn, Cr, Rb.
-
State how the oxidizing strength of each of the following oxidizing agents would be affected by raising the pH (stronger, weaker, or no change): (a) Br 2 ; (b) MnO 4 ; (c) NO 3 ; (d) ClO 4 ; (e)...
-
Consider an underground limestone mine in flat strata where pillars are 29 m high and depth is 258 m. Rock properties are: E = 77.9GPa, v = 0.20, Co = 148 MPa, To = 11.6 MPa, unit weight = 24.7kN/m3....
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
sample letter for signature change on bank accounts for principals of school
-
Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the variable cost volume variances based on a comparison between...
-
Suppose we have samples of five men and of five women and have conducted a randomization test to compare the sexes on the variable Y = pulse. Further, suppose we have found that in 120 out of the 252...
-
A heat engine has a heat input of 3 Ã 104 Btu/h and a thermal efficiency of 40 percent. Calculate the power it will produce, in hp. Source 3 x 10 Btu/h 40% HE Sink
-
Draw the mechanism of the following reaction, which involves two consecutive Friedel-Crafts alkylations. When drawing the mechanism, do not try to draw the two alkylations as occurring simultaneously...
-
A Friedel-Crafts alkylation is an electrophilic aromatic substitution in which the electrophile (E + ) is a carbocation. In previous chapters, we have seen other methods of forming carbocations, such...
-
Identify whether each of the following compounds can be made using a direct Friedel-Crafts alkylation or whether it is necessary to perform an acylation followed by a Clemmensen reduction to avoid...
-
Jen bought 100 shares of ABC stock at $15 a share on July 14, 2017. On August 7, 2018, she noticed that the stock had increased in value to $20 a share and decided to sell her shares. Jen's marginal...
-
Alex. Inci, buys 40 petcent of Steinbart Company on January 1, 2020, for $1.212.000. The equity method of accounting is to be used. Steinbart's net assets on that datewere $2.90 million. Any excess...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2

Study smarter with the SolutionInn App


