Which of the following can be classified as reactions between Brnsted acids and bases? For those that
Question:
Which of the following can be classified as reactions between Brønsted acids and bases? For those that can be so classified, identify the acid and the base.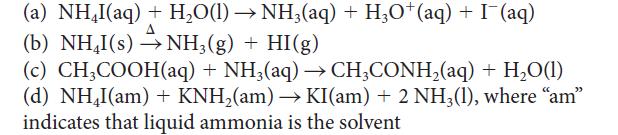
Transcribed Image Text:
(a) NHI(aq) + H,O(l)→NH,(aq) + HgO*(aq) +I(aq) (b) NH₂I(s) →→NH3(g) + HI(g) (c) CH3COOH(aq) + NH3(aq) → CH3CONH₂(aq) + H₂O(1) (d) NH₂I(am)+ KNH₂(am) → KI(am) + 2 NH3(1), where "am" indicates that liquid ammonia is the solvent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a proton transferred from NH4 to HO ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions can be classified as reactions between Brnsted acids and bases? For those that can be so classified, identify the acid and the base. (a) KOH(aq) + CH3I(aq) CH3OH(aq)...
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
The comparative balance sheets for Karidis Ceramics, Inc., for December 31, 209 and 208 are presented on the next page. During 209, the company had net income of $96,000 and building and equipment...
-
1. The Woody Company manufactures slippers and sells them at $10 a pair. Variable manufacturing cost is $4.50 a pair, and allocated fixed manufacturing cost is $1.50 a pair. It has enough idle...
-
The following data represent the final grades obtained by 5 students in mathematics, English, French, and biology. Test the hypothesis that the courses are of equal difficulty. Use a P-value in your...
-
The is the point at which the contractor assumes total responsibility for each additional dollar of contract cost. a. breakeven point b. Share Ratio Point c. Point of Reconciliation d. Point of Total...
-
The accounting records of Anderson Inc. show the following data for 2008. 1. Life insurance expense on officers was $9,000. 2. Equipment was acquired in early January for $200,000. Straight-line...
-
The maturity value of a $236,400, 9%, 40-day note receivable dated July 3 is A $236,400 B $245,856 C $257,676 D $238,764
-
Recall from Exercise 14.5 that Enterprise Industries has observed the historical data in Table 14.5 concerning y (demand for Fresh liquid laundry detergent), x1 (the price of Fresh), x2 (the average...
-
You are an environmental chemist studying a local waterway and need to know the amounts of all forms of phosphate in the stream. You might begin by making up some solutions of phosphoric acid to use...
-
You continue exploring different combinations of electrodes in pursuit of better batteries. You know that the Ce 4+ /Ce 3+ couple has been used in combination with Zn 2+ /Zn to develop new power...
-
Explain the Internal Revenue Code Sec. 148 rule on arbitrage.
-
Time ( s ) Velocity ( m / s ) 1 2 3 4 5 6 7 8 Calculate the velocity
-
The table below gives the data about Etruria's balance of payments. (All figures are in billions of dollars.) Foreign investment in Etruria Secondary (transfers) income received from abroad Primary...
-
Olive Corporation buys a material for P20 per unit. Sixteen thousand parts a year are needed. Carrying costs is P3.00 per unit and the ordering cost is P15. Required: Compute the economic order...
-
As a healthcare leader or manager, most of us are charged with supervising employees. The literature suggests the importance of hiring and retaining employees with high levels of emotional...
-
7-8. Evaluate the sum exactly. (10 points each) 7. 18 (1) n (33) "
-
Refer to the distribution of centipedes given in Exercise 3.S.2. Suppose five squares are chosen at random. Find the probability that three of the squares contain centipedes and two do not. Exercise...
-
Dan and Diana file a joint return. Dan earned $31,000 during the year before losing his job. Diana received Social Security benefits of $5,000. a. Determine the taxable portion of the Social Security...
-
Identify the reagents you would use to achieve the following transformation:
-
Propose an efficient synthesis for each of the following compounds using the malonic ester synthesis. (a) (b) (c) (d) (e)
-
Starting with diethyl malonate and using any other reagents of your choice, proposean efficient synthesis for each of the following compounds: (a) (b) (c) .
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


