A buffer is 0.100 M in NH 4 Cl and 0.100 M in NH 3 . When
Question:
A buffer is 0.100 M in NH4Cl and 0.100 M in NH3. When a small amount of hydrobromic acid is added to this buffer, which buffer component neutralizes the added acid?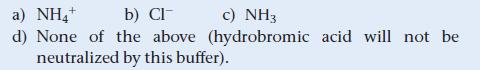
Transcribed Image Text:
a) NH4+ b) Cl c) NH3 d) None of the above (hydrobromic acid will not be neutralized by this buffer).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
c...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a small amount of iodine is added to a mixture of chlorine and methane, it prevents chlorination from occurring. Therefore, iodine is a free-radical inhibitor for this reaction. Calculate Ho...
-
A solution is 0.0500 M in NH4Cl and 0.0300 M in NH3. Calculate its OH2 concentration and its pH (a) Neglecting activities. (b) Taking activities into account.
-
1) Which of the following solutions is a good buffer system? Which of the following solutions is a good buffer system? A solution that is 0.10 M HCN and 0.10 M LiCN A solution that is 0.10 M NaCl and...
-
In Problems 530, a. Classify the sequences as arithmetic, geometric, Fibonacci, or none of these. b. If arithmetic, give d; if geometric, give r; if Fibonacci, give the first two terms; and if none...
-
Many politicians, scientists, economists, and businesspeople have become concerned about the potential implications of global warming. The largest source of the emissions thought to contribute to...
-
Identify one erosional landform produced by running water.
-
What should we do and what should we buy?
-
The accounting department supplied the following data in reconciling the September 30 bank statement for Clegg Auto. Ending cash balance per bank . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
What is the correct journal for an electricity payment of $500? Debit Credit Not applicable Cash Expense Prepayment
-
Which solution is a buffer? (a) A solution that is 0.100 M in HNO 2 and 0.100 M in HCl (b) A solution that is 0.100 M in HNO 3 and 0.100 M in NaNO 3 (c) A solution that is 0.100 M in HNO 2 and 0.100...
-
Calculate the pH of a buffer solution that is 0.100 M in HC 2 H 3 O 2 and 0.100 M in NaC 2 H 3 O 2 .
-
Preferred stock is a hybrid security; it has some of the characteristics of stock and some of the characteristics of bonds. Historically, preferred stock has not been a popular means of financing. In...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
9. For diatomic molecules, the correct statement(s) about the molecular orbitals formed by the overlap of two 2pz orbitals is (are) (A) orbital has a total of two nodal planes. (B) * orbital has one...
-
12. The treatment of galena with HNO3 produces a gas that is (A) paramagnetic (C) an acidic oxide (B) bent in geometry (D) colorless
-
The voltage across a load and the current through it are given by v(t) = 20 + 60 cos 100t V i(t) = 1 - 0.5 sin 100t A Find: (a) The rms values of the voltage and of the current (b) The average power...
-
Show that every group G with identity e and such that x * x = e for all x G is abelian.
-
A pitot-static tube is inserted into a pipe carrying methyl alcohol at 25C. A differential manometer using mercury as the gage fluid is connected to the tube and shows a deflection of 225 mm....
-
A flow nozzle similar to that shown in Fig. 15.4 is used to measure the flow of water at 120F. The pipe is 6-in Schedule 80 steel. The nozzle diameter is 3.50 in. Determine the pressure difference...
-
Select a long-throated flume from Table 14.5 that will carry a range of flow from 50 m 3 /h to 180 m 3 /h. Compute the head for each of these flows and then compute the flow that would result from...
-
i just need anssers for G,h1,h2,h3 120 a. If the opportunity cost of capital is 11%, which of these two projects would you accept (A, B, or both)? b. Suppose that you can choose only one of these two...
-
In using Verizon Communications Inc as a case analysis, what is their product portfolio, competitors and competitive Environment?/
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...

Study smarter with the SolutionInn App


