Balance each chemical equation. a. NaS(aq) + Cu(NO3)2(aq) b. NH(1) NH3(g) + N(g) c. HCl(aq) + O(g)
Question:
Balance each chemical equation.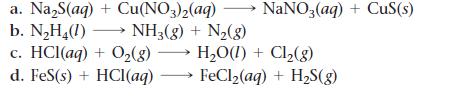
Transcribed Image Text:
a. Na₂S(aq) + Cu(NO3)2(aq) b. N₂H₂(1)→→→ NH3(g) + N₂(g) c. HCl(aq) + O₂(g) → H₂O(1) + Cl₂(g) d. FeS(s) + HCl(aq) FeCl₂(aq) + H₂S(g) NaNO3(aq) + CuS(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a NaSaq CuNO32aq NaNO3aq CuSs Balanced equation 2NaSaq ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Exercises 1-3, balance the chemical equation for each reaction.
-
The scatterplot shows the median weekly earning (by quarter) for men and women in the United States for the years from 2005 through 2017. The correlation is 0.974. a. Use the scatterplot to estimate...
-
Total current assets TOTAL ASSETS SHAREHOLDERS' EQUITY AND LIABILITIES: Shareholders' Equity: Preferred stock Common stock: Tk. 10 par 100,000 shares Share Premium 1690 2070 3060 3520 100 100 1000...
-
Transfer pricing is a significant area of concern for taxing authorities and multinational entities (MNE). Examine at least two (2) potential transfer pricing issues that create concern for both...
-
What is the yield to maturity for the bond issued by Xenon Inc.?
-
A 4-L pressure cooker has an operating pressure of 175 kPa. Initially, one-half of the volume is filled with liquid and the other half with vapor. If it is desired that the pressure cooker not run...
-
Fish Taco Company (FTC) is planning to open a taco store near new university campus housing. FTC estimates that the current neighborhoods fast-food market is $200,000 per year, which will double when...
-
Reel Time distributes DVDs to movie retailers, including dot-coms. Reel Times top management meets monthly to evaluate the companys performance. Controller Jairo Munoz prepared the following...
-
An office building generates the cash flows listed below. The owner is considering spending $55,000 to renovate the building. The renovations will change the cash flows as listed below. Calculate the...
-
1. determine the taxable income if the taxpayer is a resident citizen 2. Determine the taxable income if the taxpayer is a resident alien. 3. Determine the taxable income if the taxpayer is a...
-
Consider the unbalanced equation for the combustion of hexane: Balance the equation and determine how many moles of O 2 are required to react completely with 7.2 moles of C 6 H 14 . C6H14(g) + O(8)...
-
Write the balanced chemical equation for the reaction of aqueous potassium hydroxide with aqueous iron(III) chloride to form solid iron(III) hydroxide and aqueous potassium chloride.
-
An employee of a bear park in Montana smoked marijuana on his way to work. Later in the day, the employee went to feed the bears. The employee was subsequently mauled by a large grizzly bear and...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Wooly hair is a rare dominant trait found in people of Scandinavian descent in which the hair resembles the wool of a sheep. A male with wooly hair, who has a mother with straight hair, moves to an...
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
Picric acid is a military explosive formed via the nitration of phenol under conditions that install three nitro groups. Draw the structure and provide an IUPAC name for picric acid.
-
In each case, identify the most likely position at which monobromination would occur. (a) (b) (c) (d) N.
-
Identify the carboxylic acid and the alcohol that are necessary in order to make each of the following compounds via a Fischer esterification: a. b. c. CH 3 CH 2 CO 2 C (CH 3 ) 3
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 620 units @ $55 per unit $34,100 Purchases: January 10: 620 units @ $60 per unit January 20: 1,240 units...
-
QUESTION 1 ( 2 0 Marks ) A company manufactures and sells a single product. Budgeted data per unit of the product is: \ table [ [ , R 1 . 1 Prepare statements of Comprehensive income for both periods...
-
12. Accrual for product warranty liability could result in Future Future Taxable Amounts Deductible Amounts a. Yes Yes b. Yes No c. No Yes d. No No 13. Subscriptions received in advance could result...

Study smarter with the SolutionInn App


