Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic
Question:
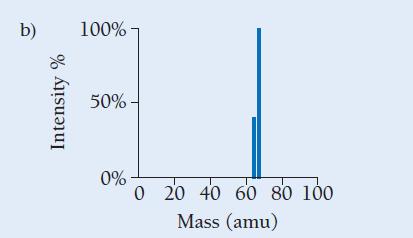
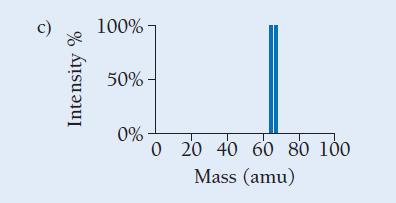
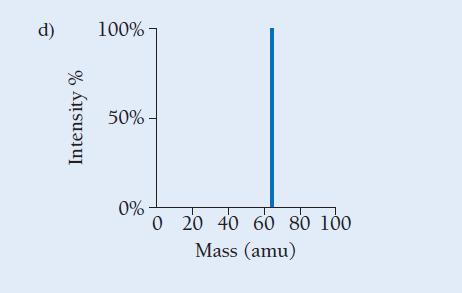
Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic mass of 63.55 amu. Which mass spectrum (of those shown at right) is most likely to correspond to a naturally occurring sample of copper?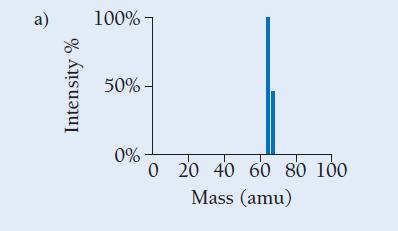
Transcribed Image Text:
a) Intensity % 100% 50% 0% 0 20 40 60 80 100 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Intensi...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An element has two naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 49.9472. 2.500 103 50.9440. 0.9975 What is the atomic mass of this...
-
(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28 Si, 27.9769265325 u, 92.223%; 29 Si, 28.976494700 u, 4.685%; 30 Si, 29.973377017 u, 3.092%....
-
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu. (a) How many protons and neutrons are in the nucleus of each isotope? Write the complete atomic symbol...
-
Steve and Linda Hom live in Bartlesville, Oklahoma. Two years ago, they visited Thailand. Linda, a professional chef, was impressed with the cooking methods and the spices used in the Thai food....
-
Star City is considering an investment in the community center that is expected to return the following cash flows: Year Net Cash Flow 1 . . . . . . . . . . . . $ 20,000 2 . . . . . . . . . . . ....
-
Consider a hedge fund whose annual fee structure has a fixed fee and an incentive fee with a high-water mark provision. The fund manager earns an incentive fee only if the fund is above the...
-
Helen Ogarth is preparing her accounts for the year to 31 December 2002. On 1 January 2002 she purchased the following fixed assets. 2 Buildings 100,000 Machine 50,000 Motor van 20,000 She wishes to...
-
Yaovi Akpawu operates Yaovis Cricket Farm in Thunder Bay, Ontario. Yaovis raises about 18 million crickets a month. Most are sold to pet stores at $12.60 for a box of 1,000 crickets. Pet stores sell...
-
Question 1 (44 marks) $ Hollings Company, a furniture manufacturer, is considering investing in some new production machinery. The details of this investment are: Amount of Investment $ 300,000...
-
Given the circuit in Fig. 3.53, calculate the currents i1 through i4? Figure 3.53 2A 0 IA
-
Explain Millikans oil drop experiment and how it led to the measurement of the electrons charge. Why is the magnitude of the charge of the electron so important?
-
A thief uses a can of sand to replace a solid gold cylinder that sits on a weight-sensitive, alarmed pedestal. The can of sand and the gold cylinder have exactly the same dimensions (length = 22 and...
-
The rms speed of the atoms in a 2.0 g sample of helium gas is 700 m/s. What is the thermal energy of the gas?
-
Identify each fringe benefit provided to Maggie and determine whether an exemption applies. (6 marks) Question 2: Explain the impact the fringe benefits will have on Maggie's taxable income and/or...
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
What is reproductive cloning? Are identical twins in humans considered to be clones? With regard to agricultural species, what are some potential advantages to reproductive cloning?
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
Calculate H and S if the temperature of 1.75 moles of Hg(l) is increased from 0.00 o C to 75.0 o C at 1 bar. Over this temperature range, C P,m = (J K -1 mol -1 ) 30.093 4.944 10 -3 T/K.
-
Draw the mechanism for each of the following reactions: a. b. c. NaOMe CI NaOEt. Br
-
In the next chapter, we will learn a method for preparing alkynes (compounds containing C ¡ C triple bonds). In the following reaction, a dihalide (a compound with two halogen atoms) is treated...
-
During 2020, PC Software Inc. developed a new personal computer database management software package. Total expenditures on the project were $5,400,000, of which 40% occurred after the technological...
-
In five years, Kent Duncan will retire. He is exploring the possibility of opening a self-service car wash. The car wash could be managed in the free time he has available from his regular...
-
EN CARE LE MULUUN Help Save A check issued for $890 to pay a vendor on account was recorded in the firm's records as $980, the canceled chock was property listed on the tank statement at $890. To...

Study smarter with the SolutionInn App


