What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
Question:
What are the formal charges of the atoms shown in red?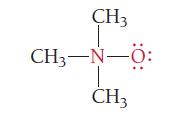
Transcribed Image Text:
CH3 CH3−N–0: -N- CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
N has a fo...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the formal charges of the atoms shown in red? :: CH3-S-CH3
-
In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H--- Ne). Identify all six elements so that the formal charges of all atoms are...
-
Consider the following separate cases pertaining to Audit Opinions: (a) You have audited Froggy No 1 Ltd. During the audit you discover that a material amount of inventory is held in a robotic...
-
Scandinavo Ltd. is a CCPC that began operations on January 1, 2020 when it was first incorporated and a calendar fiscal period was chosen. Scandinavo Ltd. Is not associated with any other...
-
Why would a company be interested in creating a data warehouse? Why would a company not be interested in creating a data warehouse?
-
TheCOVID-19pandemichascreatedopportunitiesandchallengesforbusinessesinmany countries. GAP istryingtorestructurethecompanyforthefuture.WallStreetArticle,"CanGap...
-
Direct materials price, efficiency, mix and yield variances. (Chapter Appendix) Greenwood, Inc., manufactures apple products such as apple jelly and applesauce. It makes applesauce by blending...
-
Mary Walker, president of Rusco Products, considers $14,000 to be the minimum cash balance for operating purposes. As can be seen from the following statements, only $8,000 in cash was available at...
-
A7X Co. has an ROA of 8 percent and a payout ratio of 31 percent. What is its internal growth rate? (Do not round intermediate calculations and enter your answer as a percent rounded to 2 decimal...
-
On March 1, 2023, VisionTech Inc.s board of directors declared a 5% share dividend when the market price per share was $10.00. On November 15, 2023, the board of directors declared a 4:1 share split....
-
Write the Lewis structure for each molecule (octet rule not followed). a. BCl 3 b. NO 2 c. BH 3
-
Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge number...
-
Answer the following questions about prepaid expenses: a. On November 1, Air & Sea Travel prepaid $3,000 for 6 months' rent. Give the adjusting entry to record rent expense at December 31. Include...
-
Pascall Company has the following information available for the past year: The companys hurdle rate is 10 percent. 1. Determine Pascalls return on investment (ROI) and residual income for each...
-
A company allocates overhead using direct labor hours. The expected number of direct labor hours is 4,000 for the coming year and the expected overhead is \($80,000.\) During the year, the company...
-
Reproduce the plots in Fig. 29.10 by deriving formulas for the eigenvalues and eigenfunctions of the Hamiltonian (29.4). Hint: See the solution of Problem 14.14 . Data from Fig. 29.10 4 2- E OF -2...
-
Sketch the results of path multiplications (indicated by \(\times\) ) for these examples: O (a) (b) (c) where each loop is defined in the same 2D euclidean plane with a single hole.
-
Assume the economy is in equilibrium. Analyze the effect of a cut in autonomous expenditure on economic activity and the level of unemployment. You should use a diagram to help illustrate your answer.
-
A brass alloy to be used for a spring application must have a modulus of resilience of at least 0.75 MPa (110 psi). What must be its minimum yield strength?
-
Calculate the number of neutrons of 239Pu.
-
For the differential manometer shown in Fig. 3.27, calculate the pressure difference between points A and B. The specific gravity of the oil is 0.85. 10 in 32 in Oil Water 9 in el
-
For the manometer shown in Fig. 3.28, calculate (p A - p B ). Oil (sg = 0.85) 8 in Water 33 in 12 in
-
For the manometer shown in Fig. 3.29, calculate (p A - p B ). Oil l 150 mm (sg = 0.90) Water 750 mm Mercury (sg = 13.54) 500 mm
-
Use technology and the future value formula to find FV = $3,479; PMT = $300; n = 9; i = ? i = (Round to two decimal places as needed.)
-
In this lesson, we learned that almost all capital budgeting decisions faced by the firm contain embedded real options. Discuss ways that you can apply "real options" analysis to everyday decisions...
-
, Sws T- ta , Sws T- ta

Study smarter with the SolutionInn App


