What are the formal charges of the atoms shown in red? :: CH3-S-CH3
Question:
What are the formal charges of the atoms shown in red?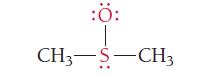
Transcribed Image Text:
:Ö: CH3-S-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The formal charges of the atoms shown in red in the image are 0 Formal charge i...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
-
In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H--- Ne). Identify all six elements so that the formal charges of all atoms are...
-
Consider the following separate cases pertaining to Audit Opinions: (a) You have audited Froggy No 1 Ltd. During the audit you discover that a material amount of inventory is held in a robotic...
-
Tony acquired 1,000 shares in X Co (a resident public company) for $10 each in August 2000. In January this year X Co returned $7 of capital to its shareholder in respect to each share they held. The...
-
Discuss both the advantages and disadvantages of using a computerized database system rather than a manual system for storing and processing accounting data. In your discussion, provide some specific...
-
Merger Valuation with Change in Capital Structure Hasting Corporation estimates that if it acquires Vandell Corporation, synergies will cause Vandells free cash flows to be $2.5 million, $2.9...
-
Revenue allocation, bundled products. Athletic Programs (AP) sells exercise videos through television infomercials. It uses a well-known sporting celebrity in each video. Each celebrity receives a...
-
Methanol is added to a storage tank at a rate of 1200 kg/h and is simultaneously withdrawn at a rate mw (t) (kg/h) that increases linearly with time. At t = 0 the tank contains 750 kg of the liquid...
-
Read the announcement by Tesla (NASDAQ: TSLA) below: Tesla Announces a Five-for-One Stock Split GLOBE NEWSWIRE, Aug 11, 2020 PALO ALTO, Calif., Aug. 11, 2020 (GLOBE NEWSWIRE) -- Tesla, Inc. announced...
-
It is October 16, 2014, and you have just taken over the accounting work of China Moon Products, whose annual accounting period ends October 31. The company's previous accountant journalized its...
-
Write the Lewis structure for each molecule (octet rule not followed). a. BCl 3 b. NO 2 c. BH 3
-
Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge number...
-
(a) Prove that h(s) defined by Is an symmetric tensor. (b) Prove that h(A) defined by Is an antisymmetric tensor. (c) Find the components of the symmetric and antisymmetric parts of defined in Exer....
-
Refer to the information presented in E10-8 for Easy Roller. Required: Prepare the journal entry to record the following for Easy Roller: 1. Direct materials costs and related variances. Assume the...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Supplies Expense Cash Equipment Common Stock Dividends Building...
-
Determine if Heards statements are correct. Justify your response. Laura Powers is a senior investment analyst at Brotley University Foundation and works for the university endowment. Powers is...
-
Determine the most appropriate rebalancing choice for the Foundations investment team. Justify your response. The Lemont Family Foundation follows a systematic quarterly rebalancing policy based on...
-
The following account balances, in alphabetical order, are from the general ledger of Milo's Waterproofing Service at January 31. The firm began business on January 1 . All accounts have normal...
-
Show that Equations 6.18a and 6.18b are valid when there is no volume change during deformation.
-
Define the essential properties of the following types of operating systems: a. Batch b. Interactive c. Time sharing d. Real time e. Network f. Parallel g. Distributed h. Clustered i. Handheld
-
Figure 3.24 shows a closed container holding water and oil. Air at 34 kPa below atmospheric pressure is above the oil. Calculate the pressure at the bottom of the container in kPa(gage). 0,25 m Air...
-
Determine the pressure at the bottom of the tank in Fig. 3.25. 1.2 m --- --- -- -- Air 200 kPa (gage) Oil 15m/ (sg = 0.80) 2.6 m Water 2 m
-
Water is in the pipe shown in Fig. 3.26. Calculate the pressure at point A in kPa(gage). Pipe 100 mm - Water 75 mm Mercury (sg = 13.54)
-
You are considering the purchase of new living room furniture that costs $1,180. The store will allow you to make weekly payments of $25.89 for one year to pay off the loan. What is the EAR of this...
-
Question 17 (2 points) An increase in assets: Increases income Does not affect cash Increases cash Reduces cash
-
Martell Mining Companys ore reserves are being depleted, so its sales are falling. Also, because its pit is getting deeper each year, its costs are rising. As a result, the companys earnings and...

Study smarter with the SolutionInn App


