Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each
Question:
Draw the Lewis structure (including resonance structures) for methyl azide (CH3N3). For each resonance structure, assign formal charges to all atoms that have formal charge.
Transcribed Image Text:
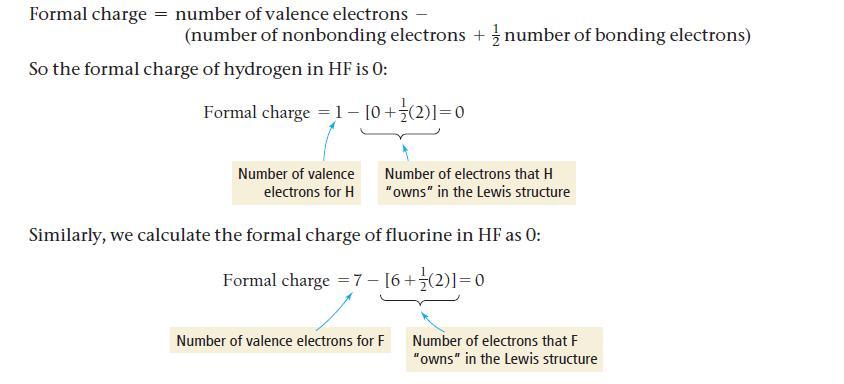
Formal charge number of valence electrons - (number of nonbonding electrons + number of bonding electrons) So the formal charge of hydrogen in HF is 0: Formal charge = 1- [0+ (2)]=0 Number of valence electrons for H Number of electrons that H "owns" in the Lewis structure Similarly, we calculate the formal charge of fluorine in HF as 0: Formal charge = 7 - [6+ (2)] = 0 Number of valence electrons for F Number of electrons that F "owns" in the Lewis structure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
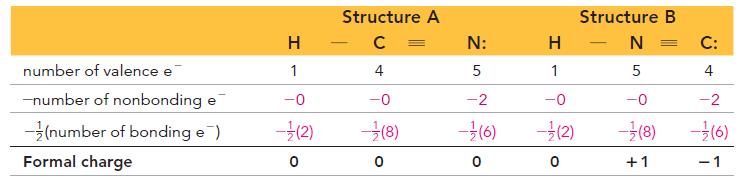
H HCNNN H H HCNNN H I II For structureI Formal charge for all the three Hatom...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure (including resonance structures) for the acetate ion (CH 3 COO ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge =...
-
Draw the Lewis structure (including resonance structures) formethyl azide (CH3N3). For each resonance structure, assign formalcharges to all atoms that have formal charge. Draw the Lewis dotstructure...
-
Draw the Lewis structure (including resonance structures) for nitromethane (CH 3 NO 2 ). For each resonance structure, assign formal charges to all atoms that have formal charge.
-
Problem 3: Dorado and Maya are the children of Jess and Sabel. In November 2000 Sabel died intestate leaving P2,000,000 estate before P1,000,000 deductions and P15,000 estate tax. How would the...
-
What are data warehouses? How are they like databases? How do they differ from databases?
-
Suppose your firm could purchase another firm for only half of its replacement value. Would that be a sufficient justification for the acquisition? Why or why not?
-
Customer profitability, distribution. Figure Four is a distributor of pharmaceutical products. Its activity-based costing system has five activity areas: Activity Area Cost Driver and 2007 Rate 1....
-
Krall Company was formed on January 1, 2014, and began constructing a new plant. At the end of 2014, its auditor discovered that all expenditures involving long-term assets had been debited to an...
-
Use the following information to prepare a budgeted income statement for Miller Company for the month of June. a. Beginning cash balance on June 1 is $62,000. b. Sales amounts are: April (actual),...
-
Newark Plastics Corporation developed its overhead application rate from the annual budget. The budget is based on an expected total output of 720,000 units requiring 3,600,000 machine hours. The...
-
What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
Is continued operation of the business just too risky given the new competition across the street in the form of a new DSL business by the phone company and the new multichannel video company in the...
-
Verify the projection characteristics implied by equations (14.51). Data from Eq.14.51 2 In = (s + I) Da 1- 24(1-y's) = UR
-
Evaluate the following statement: Every population with individual values making up a total value has a population mean, population standard deviation, and population distribution. The population...
-
Carry out an environmental scan of an organization you know well. The following steps should help: (a) Using the PEST framework, the results for one of the organizations chosen for Activity 1.7 and...
-
The following questions relate to the auditor's responsibility for reporting on inconsistency of application of accounting principles. Select the best response. a. Raider uses the last-in, first-out...
-
Sketch a totem-pole output stage and explain its operation and the advantages of incorporating this circuit in the TTL circuit.
-
Determine the modulus of resilience for each of the following alloys: Use modulus of elasticity values in Table 6.1. lield Strength Material Steel alloy Brass alloy Alhmimm alloy Titanium alloy MPa...
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
For the manometer shown in Fig. 3.30, calculate (p A - p B ). Water IB 150 mm - Mercury (sg = 13.54) 900 mm Oil (sg = 0.86) 600 mm
-
For the compound manometer shown in Fig. 3.31, calculate the pressure at point A. Oil (sg = 0.90) Water 125 mm 475 mm 250 mm 50 mm Mercury (sg = 13.54)
-
For the compound differential manometer in Fig. 3.32, calculate (p A - p B ). Water, Oil (sg = 0.90) 6 in 8 in 6 in 10 in 6 in Mercury (sg = 13.54)
-
Failing states are a major problem for the the entire global community of nation-states. Why are failing states such a concern and how should countries like the United States, with both hard and soft...
-
Explain which of all the financial statements (balance sheet, income statement, cash flow statement, statement of equity, etc) is the most suitable for these three; shareholders, investor and...
-
What three options does a buyer have with non-conforming goods?

Study smarter with the SolutionInn App


