What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and
Question:
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and HNO3 are mixed?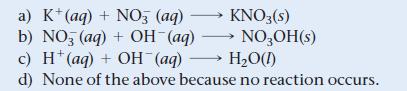
Transcribed Image Text:
a) K+ (aq) + NO3 (aq) b) NO (aq) + OH¯(aq) c) H(aq) + OH(aq) →→→ H₂O(1) d) None of the above because no reaction occurs. KNO3(s) NO3OH(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
c H...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and SrCl 2 are mixed? a) K (aq) + CI (aq) KCI(s) b) Sr+ (aq) + 2 OH-(aq) Sr(OH)2 (s) c) H* (aq) + OH (aq) ...
-
You are developing an accrual-adjusted income statement from a cash income statement. During the year, accounts payable increased. What is the adjustment you need to make?" add ending accounts...
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KHCO 3 and HBr are mixed? KCH3O (s) a) K+ (aq) + CH3O (aq) b) H+ (aq) + HCO3 (aq) - c) H+ (aq) + OH-(aq) CO(g) +...
-
1. Prepare program using threads in java that can print 10 times the numbers 1,2,3,4,5 in a series. 2. Prepare program using threads and a semaphore in java that can print the numbers 1,2,3,4,5 in a...
-
Figure is a system flowchart for P. Miesing and Companys purchase order event. Prepare a narrative to accompany the flowchart describing this purchase order event. Include in your narrative the...
-
Based on Exhibit C, the potential all-in USD return on the carry trade is closest to: A. 1.04 percent. B. 1.40 percent. C. 1.84 percent. Ed Smith is a new trainee in the foreign exchange (FX)...
-
List and explain each of the desirable elements of an insurable risk.
-
Jordan Auto has developed the following production plan for its new auto part. Each unit passes through two departments before completion. The company has developed the following direct labor...
-
E11-15 (Algo) Comparing Capital Budgeting Methods (LO 11-1, 11-2, 11-3, 11-4, 11-6] The following table contains information about four potential investment projects that Castle Corporation is...
-
Vanessa Noel, owner and manager of Noel Draperies and Window Treatments, has been receiving some complaints from her loyal clientele of interior decorators and home dcor consultants. For example, one...
-
Write complete ionic and net ionic equations for each reaction. (a) 3 SrCl (aq) + 2 Li3PO4(aq) + KOH(aq) (b) HCH3O2(aq) Sr3 (PO4)2(s) +6 LiCl(aq) HO(1) + KCH3O(aq)
-
Write an equation for the precipitation reaction that occurs (if any) when solutions of sodium nitrate and lithium sulfate are mixed.
-
Cash Flow to Stockholders and Creditors Could a companys cash flow to stockholders be negative in a given year? ( Hint: Yes.) Explain how this might come about. What about cash flow to creditors?
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
I it possible for two screw dislocations of opposite sign to annihilate each other? Explain your answer.
-
1. As a general strategy, would you recommend that Carl take an aggressive approach to capacity expansion or more of a wait-and-see approach? 2. Should Carl go with the option for one facility that...
-
Argon-ion lasers typically generate multi-watt beams in the green or blue regions of the visible spectrum. Determine the frequency of such a 514.5-nm beam.
-
Consider the function where A is a constant. Show that it is a solution of the differential wave equation. Determine the speed of the wave and the direction of propagation. = (1 '2)p (z vt) + 1
-
Show that the function is a solution of the differential wave equation. In what direction does it travel? (y, t) = (y 41)
-
Growth Corps free cash flow is $1,000,000,000 and is expected to grow by at least 4% per year for the foreseeable future. They have $2,000,000,000 in long-term debt and 100,000,000 shares...
-
You are considering the purchase of an apartment complex that will generate net cash flows each of the next 20 years, starting at $400,000 in Year 1. You normally demand a 10% rate of return on such...
-
Jackson Company produces plastic that is used for injection molding applications such as gears for small motors, In 2016, the first year of operations, Jackson produced 4,200 tons of plastic and sold...

Study smarter with the SolutionInn App


