What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and
Question:
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and SrCl2 are mixed?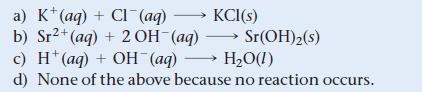
Transcribed Image Text:
a) K (aq) + CI (aq) →→→→KCI(s) b) Sr²+ (aq) + 2 OH-(aq) Sr(OH)2 (s) c) H* (aq) + OH (aq) →→→ H₂O(1) d) None of the above because no reaction occurs.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
2 b S...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and HNO 3 are mixed? a) K+ (aq) + NO3 (aq) b) NO (aq) + OH(aq) c) H(aq) + OH(aq) HO(1) d) None of the above...
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KHCO 3 and HBr are mixed? KCH3O (s) a) K+ (aq) + CH3O (aq) b) H+ (aq) + HCO3 (aq) - c) H+ (aq) + OH-(aq) CO(g) +...
-
What is the net ionic equation for the reaction that occurs when an aqueous solution of KI is added to an aqueous solution of Pb(NO 3 ) 2 ?
-
8. Be able to discuss how economic growth was differed across the three eras we discussed prior to WWI.
-
Search the web for unusual and interesting uses of RFID tags. Find at least two that are unusual and share those with your classmates.
-
If an excise tax is paid by the buyer instead of the seller, which of the following statements is most likely to be true? A. The price paid will be higher than if the seller had paid the tax. B. The...
-
Explain how the law of large numbers supports the operation of the insurance mechanism
-
Express Co. purchased equipment on March 1, 2015, for $95,000 on account. The equipment had an estimated useful life of five years, with a residual value of $5,000. The equipment is disposed of on...
-
TB MC Qu . 0 8 - 9 0 ( Algo ) Summerlin Company budgeted... Summerlin Company budgeted 4 , 9 0 0 pounds of material costing $ 6 . 0 0 per pound to produce 2 , 5 0 0 units. The company actually used 5...
-
George Pharmacy is a pharmaceutical salesman who has been very successful at his job in the last few years. Unfortunately, his family life has not been very happy. Three years ago, his only child,...
-
What are the solubility rules? How are they useful?
-
The presence of one of the ANSWER NOW! following ions within a compound indicates that a compound is soluble with no exceptions. Which ion? (a) OH (b) SO- (c) NO3
-
The board can be adjusted vertically by tilting it up and sliding the smooth pin A along the vertical guide G. When placed horizontally, the bottom C then bears along the edge of the guide, where the...
-
A pistoncylinder device contains 0.85 kg of refrigerant-134a at 210 oC. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now,...
-
3.3. Using the BEMT, show the effect of increasing linear twist on the variations in inflow, thrust, induced power, profile power, and lift coefficient across the span of a rotor with four blades of...
-
By uploading this work, I attest that the work contained herein is solely my own, that I only used the given equation sheet as a reference, and that I have not received any information from anyone...
-
Demand for patient surgery at Washington General Hospital has increased steadily in the past few years, as seen in the following table: ...
-
Explain product analysis
-
Indicate the location in the vicinity of an edge dislocation at which an interstitial impurity atom would be expected to be situated. Now briefly explain in terms of lattice strains why it would be...
-
The graph of the sequence whose general term is an = n - 1 is which of the following? [8.1] A. B. TITTT 3-2-1 23.45 2.3.4
-
Identify an actionreaction pair of forces in each of the following situations. (a) A person pushing on a wall (b) A book resting on a table (c) A hockey puck sliding across an icy surface (d) A car...
-
Figure P2.55 shows the position as a function of time for an apple that falls from a very tall tree.? (a) At what time does the apple hit the ground?? (b) Use a graphical approach to estimate and...
-
Figure P2.53 shows the position as a function of time for an object. (a) What is the average velocity during the period from t = 0.0 s to t = 10.0 s? (b) What is the average velocity between t = 0.0...
-
PART II (35 possible marks) Woolsworth Corporation manufactures and sells woolen jackets. It is ready to begin its fourth quarter, in which they have their highest sales. The company has approached...
-
1) A portfolio consists for four securities A, B, C &D have the following expected rate of return and portfolio value invested. Security Expected Return Proportion of investment A 15% 30% B 12% 20% C...
-
A firm financed by equity and debt only has financial risk. (Your answer must begin with True or False followed by your explanation.) The level of debt in a firm does not affect the firms valuation...

Study smarter with the SolutionInn App


