What is the net ionic equation for the reaction that occurs when aqueous solutions of KHCO 3
Question:
What is the net ionic equation for the reaction that occurs when aqueous solutions of KHCO3 and HBr are mixed?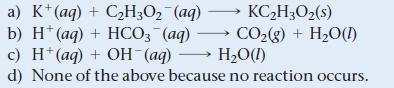
Transcribed Image Text:
KC₂H3O₂ (s) a) K+ (aq) + C₂H3O₂ (aq) b) H+ (aq) + HCO3 (aq) - c) H+ (aq) + OH-(aq) CO₂(g) + H₂O(1) H₂O(1) d) None of the above because no reaction occurs.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
b H ...View the full answer

Answered By

Allan Olal
I have vast tutoring experience of more than 8 years and my primary objective as a tutor is to ensure that a student achieves their academic goals.
4.70+
78+ Reviews
412+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and HNO 3 are mixed? a) K+ (aq) + NO3 (aq) b) NO (aq) + OH(aq) c) H(aq) + OH(aq) HO(1) d) None of the above...
-
What is the net ionic equation for the reaction that occurs when aqueous solutions of KOH and SrCl 2 are mixed? a) K (aq) + CI (aq) KCI(s) b) Sr+ (aq) + 2 OH-(aq) Sr(OH)2 (s) c) H* (aq) + OH (aq) ...
-
What is the net ionic equation for the reaction that occurs when an aqueous solution of KI is added to an aqueous solution of Pb(NO 3 ) 2 ?
-
Which statement about the pass-by-reference is NOT true? a.Every time you pass a reference variable to a method, you also pass the object referred by the refenerece variable to the method. b.In the...
-
What non-financial information would be important for an AIS to capture about a manufacturing firms production process?
-
Suppose someone says, Using monetary or fiscal policy to pump up the economy is counterproductiveyou get a brief high, but then you have the pain of inflation. a. Explain what this means in terms of...
-
Briefly describe the three general categories into which private or voluntary insurance may be divided.
-
Data for Obras Corporation in 2014 and 2013 follow. These data should be used in conjunction with the data in P1. In P1, Obras Corporations condensed comparative income statements and comparative...
-
Perpetually Preferred, Inc. (PPI) has a plain vanilla issue of preferred stock outstanding that pays a fixed annual dividend of $6.25 per share. PPI's preferred is perpetual so that it has no...
-
Company A is a global company based in the United States that operates in the financial industry. Company A serves its customers with financial products, such as checking accounts, bank cards, and...
-
How can you predict whether a precipitation reaction will occur upon mixing two aqueous solutions?
-
A 10.0 mL sample of 0.20 M HBr solution is titrated with 0.10 M NaOH. What volume of NaOH is required to reach the equivalence point? (a) 10.0 mL (b) 20.0 mL (c) 40.0 mL
-
Pineapple Beach Hotel Co. (Pineapple) operates a hotel providing accommodation, leisure facilities, and restaurants. Its year end was April 30, 2020. You are the audit senior of Berry & Co. and are...
-
in a thermodynamics, a phase means what?
-
Give me a 3 python codes in (Discrete Mathematics course) for : 1-basic algorithm 2- the growth of functions 3- Complexity of Algorithms And explain how its works with examples.
-
Using the perpetual inventory system, calculate the ending inventory and Cost of Goods Sold under each of the following methods. Beginning Inventory 10 units @ $1 Purchases January 5 January 20 20...
-
the manager of wongs food express estimates operating costs for the year will total $300,000 for fixed costs. 28. find the sales dollars required with a contribution margin ratio of 40 percent to...
-
Tara Williams and Tilly North had been yoga buddies for almost a decade. Yoga was an escape from the daily stresses of being working parents for both of them. One thing Tara and Tilly always talked...
-
The average grain diameter for a brass material was measured as a function of time at 650°C, which is tabulated below at two different times: (a) What was the original grain diameter? (b) What...
-
Vince, Inc. has developed and patented a new laser disc reading device that will be marketed internationally. Which of the following factors should Vince consider in pricing the device? I. Quality of...
-
Consider the pulse described in terms of its displacement at t = 0 by where C is a constant. Draw the wave profile. Write an expression for the wave, having a speed v in the negative x-direction, as...
-
Write the expression for the wavefunction of a harmonic wave of amplitude 10 3 V/m, period 2.2 10 -15 s, and speed 3 10 8 m/s. The wave is propagating in the negative x-direction and has a value of...
-
Show that if the displacement of the string in Fig. 2.12 is given by y(x, t) = A sin [kx - Ït + ε] then the hand generating the wave must be moving vertically in simple harmonic...
-
Adjusting entries ABC. Co purchased 600 of office supplies on account on October 15th. On December 31st, 150 of office supplies remain on hand. Prepare the journal entries to record the October 15th...
-
Calculate the present value of cash flows, 1500 in the year 1 then grows at 2% every year, using 10% discount rate. Round and write up to two decimals (e.g., 100.00). No characters including comma...
-
The most recent statement of financial position and statement of comprehensive income of Lucky Inc. are shown here: Statement of Financial Position Recent Recent ASSETS LIABILITIES & OWNERS' EQUITY...

Study smarter with the SolutionInn App


