A 5.00-g sample of one of the substances listed in Table 7.1 was heated from 25.2C to
Question:
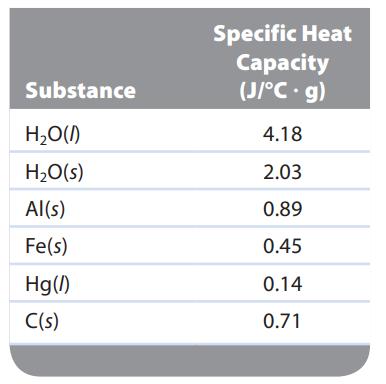
A 5.00-g sample of one of the substances listed in Table 7.1 was heated from 25.2°C to 55.1°C, requiring 133 J to do so. Which substance was it?
Table 7.1
Transcribed Image Text:
Substance H₂O(l) H₂O(s) Al(s) Fe(s) Hg(/) C(s) Specific Heat Capacity (J/°C. g) 4.18 2.03 0.89 0.45 0.14 0.71
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To determine which substance was heated we can use the following ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Let n=102 +r, where , re N, 0r9. A number a is chosen at random from the set {1, 2, 3, ..., n} and let pn denote the probability that (a 1) is divisible by 10. 70. If r = 0, then npn equals (a) 22...
-
Falcon Company incurs a $18 per unit cost for Product A, which it currently manufactures and sells for $27 per unit. Instead of manufacturing and selling this product, the company can purchase...
-
A market research company surveyed consumers to determine their ranked preferences of energy drinks among the brands Monster, Red Bull, and Rockstar. a. What are the outcomes of this experiment for...
-
What is the degree of centralisation versus decentralisation? LO1
-
Top managers of Stenback Industries predicted 2016 sales of 14,900 units of its product at a unit price of $7.00. Actual sales for the year were 14,300 units at $10.50 each. Variable costs were...
-
3. Masulis Inc. is considering a project that has the following cash flow and WACC data. What is the project's discounted payback? 3 $445 4 S405 S485 WACC: 10.00% Year 0 Cash flows -$1,450 $525 a....
-
Four key organizational complements must be in place to ensure successful implementation and use of a new system. Which two of these components seem to be missing at your store?
-
In a coffee-cup calorimeter, 100.0 mL of 1.0 M NaOH and 100.0 mL of 1.0 M HCl are mixed. Both solutions were originally at 24.6C. After the reaction, the final temperature is 31.3C. Assuming that all...
-
Are the following processes exothermic or endothermic? a. the combustion of gasoline in a car engine b. water condensing on a cold pipe c. CO (s)- CO(g) d. F(g) 2F(g)
-
Name two types of electrolytic capacitors. How do electrolytics differ from other capacitors?
-
Given the following differential equation, dydx = sin ( x + y ) Find the following: ( a ) The substitution u = ( b ) The transformed differential equation dudx = ( c ) The implicit solution, given...
-
Consider the following type declarations TYPE Alinteger; A2 pointer to float; A3 pointer to integer; T1 structure (x: integer; } T2 structure (x: A1; next pointer to integer; } b float; } a :...
-
https://www.viddler.com/embed/82b62f65 Questions: How do companies decide where to locate their facilities? Why has just-in-time inventory control become a dominant production process used in the...
-
Adjusting Entries for Interest At December 31 of Year 1, Portland Corporation had two notes payable outstanding (notes 1 and 2). At December 31 of Year 2, Portland also had two notes payable...
-
We want to get an idea of the actual mass of 235U involved in powering a nuclear power plant. Assume that a single fission event releases 200 MeV of thermal energy. A 1,000 MWe electric power plant...
-
What limits the cross-sectional area of most P/M parts to several square inches or less?
-
a) Show that (a, b) := {{a}, {b}} does not satisfy the ordered pair axiom. b) Determine whether each of the following statements is true or false. (Give a reason in each case): (i) {a, b} C (a, b)....
-
Draw a bond-line structure for each of the following compounds: a. 3-Isopropyl-2, 4-dimethyl-2-pentene b. 4-Ethyl-2-methyl-2-hexene c. 1, 2-Dimethylcyclobutene (The name of a cycloalkene will not...
-
Show how you would use a Grignard reaction to prepare each compound below. a. b. c. d. e. f. OH
-
In the mass spectrum of bromobenzene (Figure 15.27), the base peak appears at m/z = 77. Figure 15.27 a) Does this fragment contain Br? Explain your reasoning. b) Draw the cationic fragment that...
-
Excel Online Structured Activity: Replacement Analysis The Gilbert Instrument Corporation is considering replacing the wood steamer it currently uses to shape guitar sides. The steamer has 6 years of...
-
On January 1, 20X1, Toy inc. issued $500,000 of convertible bonds. The bonds mature on December 31, 20X5. Interest is payable annually at 6.0% on December 31. The bonds are convertible at the...
-
There is a bond on a companys books with an original term of 10 years that was purchased for a premium at its issuance, just over 2 years ago. The bond pays semi-annual interest. With the receipt of...

Study smarter with the SolutionInn App


