Without using Fig. 3.4, predict which bond in each of the fol- lowing groups will be the
Question:
Without using Fig. 3.4, predict which bond in each of the fol- lowing groups will be the most polar.
Data in Fig. 3.4
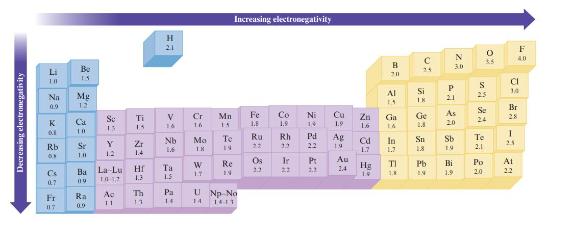
a. C-F, Si-F, Ge-F
b. P-Cl or S-Cl
c. S-F, S-Cl, S-Br
d. Ti-Cl, Si-Cl, Ge-Cl
Transcribed Image Text:
Decreasing electronegativity Li 18 K Na Mg 0.9 12 X 0. Cs 07 Be 20 Fr 117 Rb Se Y 1.2 de ²2:29 Ca 10 1.0 Ba 019 Ra Sc 13 0.9 Ti 15 Ac 11 Zr 1.4 La-Lut 10:12 1.3 = Th 14 H 21 V Nb 23 21 4: Hf Ta Pa 14 Cr 16 Mo IX W e p 1.7. Mn 14 To 19 Increasing electronegativity Re IN U Np No 14 1411 Fe 1.8 Ru 2# 8A 22 Os Co Ni 1.9 2:23 E Ir 22 14 Rh Pd 33 Pt 2.3 Cu 19 Ag 19 Au 24 398 25 1.6 Cd 1.7 B Zn Ga 14 Hg F 201 28 F3 TI с Si IK 33 1.8 Pb 30 27 29 82 N 21 As Sn Sb 18 20 19 Bi 1.9 0 S 25 Te 2.1 35 Se 24 Po 20 Cl 34 F 40 Br 28 -3 25. At 22
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To predict which bond in each of the following groups will be the most polar we can use the followin...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Without using Fig. 3.4, predict which bond in each of the following groups will be the most polar. Fig. 3.4 a. C-H, Si-H, Sn-H b. Al-Br, Ga-Br, In-Br, Tl-Br c. C-O or Si-O d. O-F or O-CI Decreasing...
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
The following data applies to the two unrelated companies Lloyd Ltd and Cole Ltd: All taxable and deductible temporary differences relate to the profit or loss. Assume a corporate tax rate of 30%. A....
-
Given that real exchange rates fluctuate, when would be the best time to enter the market of a foreign country as an exporter to that market?
-
Reconsider the sales data for a certain product given in Prob. 27.5-4. The companys management now has decided to discontinue incorporating seasonal effects into its forecasting procedure for this...
-
Restaurant and lodging chains have been quicker than independently owned and operated hospitality operations to adopt new technologies in the workplace. L01 A. True B. False
-
Recording events in T-accounts and preparing financial statements Pruitt Manufacturing Company was started on January 1, 2011, when it acquired $2,000 cash from the issue of common stock. During the...
-
PLEASE ANSWER ASAP these are the criteria Each individual item of information in a database record. Field Choose... Choose... Allows the user to add new records or scroll through data records Data...
-
Which of the following incorrectly shows the bond polarity? Show the correct bond polarity for those that are incorrect.
-
Describe the type of bonding that exists in the Cl 2 (g) molecule. How does this type of bonding differ from that found in the HCl(g) molecule? How is it similar?
-
Draw the condensed structural formula for the three isomers of pentane, C 5 H 12 .
-
Perpetual Inventory Control Record Description: M & B Supreme Date Purchase Received Issued Sales Units Unit Cost June 1 Balance forward 3 $10.00 4 2 6 8 9 $10.50 9 12 32 3 6 2 4 15 6 10 $11.00 18 20...
-
A rectangular footing of size 4m by 5m is founded at 2m below ground level in a uniform deposit of saturated clay. The footing is designed to support a total vertical load of 8000 kN inclusive of the...
-
P6.2 At the start of Tom Stoppard's "Rosencrantz and Guildenstern are dead" 1, Rosencrantz finds a coin. Guildenstern watches as Rosencrantz repeatedly tosses the coin and every time it comes down...
-
For the data: 9 5 10 7 9 10 11 8 12 769 a) Compute the z-score for the raw score of 10 b) Find the raw score that corresponds to z=+1.22
-
(11%) Problem 7: After a bad thunderstorm, a loose power line comes to rest on a parked van. The van is insulated from the ground by its tires, and accumulates an electric charge of Q = 0.0012...
-
Let X ~ n(μ, Ï2), Ï2 known. For each c ¥ 0, define an interval estimator for μ by C(x) = [x - cÏ, x + cÏ] and consider the loss in (9.3.4). a. Show...
-
When the Department of Homeland Security created a color-coded system to prepare government officials and the public against terrorist attacks, what did it do right and what did it do wrong?
-
If a solution contains either Pb 2+ (aq) or Ag + (aq), how can temperature be manipulated to help identify the ion in solution?
-
Silver chloride dissolves readily in 2 M NH 3 but is quite insoluble in 2 M NH 4 NO 3 . Explain.
-
The stepwise formation constants for a complex ion usually have values much greater than 1. What is the significance of this?
-
Assume iMost has unlimited resources (can invest in both projects), based on their break-even time, should iMost accept Projects Y and Z? What other factors should iMost consider? Project Y = 3.8 yrs...
-
BlockWorks will generate structure analysis results for all sole proprietors filing a Schedule C if the return is prepared by a small business certified Tax Preparer.
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 19,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...

Study smarter with the SolutionInn App


