Without using Fig. 3.4, predict which bond in each of the following groups will be the most
Question:
Without using Fig. 3.4, predict which bond in each of the following groups will be the most polar.
Fig. 3.4
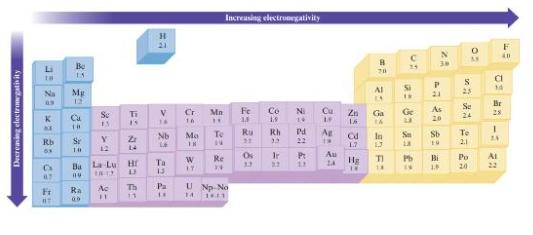
a. C-H, Si-H, Sn-H
b. Al-Br, Ga-Br, In-Br, Tl-Br
c. C-O or Si-O
d. O-F or O-CI
Transcribed Image Text:
Decreasing electronegativity Li Na * KE Rb 63 C 67 Fi #T Be 15 7 * =2 E Mg Se Y 12 Se 13 Ra 00 Ti 15 Ba La Lu Hr Bu Ac 11 Z 14 O D Th 11 H 21 V 14 Nb 23 22 4: Ta Pa Cr 16 9: Mo 18 W 17 Mn H Increasing electronegativity To 14 Re 14 U Np No 14 16.11 Fe 2= 2 82 Ru 3.3 Co 19 Rh 33 N Cu 2: 23 42 Pd 22 8: 2:33 1.3 Ag 1r P: Au 3.2 39 89 22 C 25 20 Al 18 D Ga Ge 23 In Sa 13 Zn 16. 1.7 Hg ROBS an Rd TI 14 IN a £: Pb N 30 P 21 As 20 Sb 19 Bi 1.8 0 S 23 To 2.1 AV Se 24 Po 20 a 14 Br 28 -: F 40 24 Ai
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a CH SiH SnH Carbon is more electronegative than silicon and ...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Without using Fig. 3.4, predict which bond in each of the fol- lowing groups will be the most polar. Data in Fig. 3.4 a. C-F, Si-F, Ge-F b. P-Cl or S-Cl c. S-F, S-Cl, S-Br d. Ti-Cl, Si-Cl, Ge-Cl...
-
Do you have convincing evidence of sufficient computer skills to engage in online discussion forums, access online library resources, engage in online videoconferencing, and utilize word processing,...
-
You have been asked to evaluate possible sites for an Asian production facility that will manufacture your firms products and sell them to the Asian market. What real exchange rate considerations...
-
Quality Bikes is a wholesale firm that specializes in the distribution of bicycles. In the past, the company has maintained ample inventories of bicycles to enable filling orders immediately, so...
-
Minimum wage, child labor laws, and workers rights in general have been in place in the United States since the mid-1800s. L01 A. True B. False
-
A tablet computer manufacturer has three models in its product line: The Mini model costs $375 to produce and sells for $499. 60,000 were sold. The Standard model costs $390 to produce and sells for...
-
A risk-free, zero-coupon bond has 15 years to maturity. Which of the following is closest to the price per $100 of face value that the bond wil trade at if the YTM 752 O A $32.68 OB. $29.55 OC $36.24...
-
Which of the following incorrectly shows the bond polarity? Show the correct bond polarity for those that are incorrect.
-
Describe the type of bonding that exists in the Cl 2 (g) molecule. How does this type of bonding differ from that found in the HCl(g) molecule? How is it similar?
-
Quality control has been a problem with a new product assembly line, and a multiple regression analysis is being used to help identify the source of the trouble. The daily ?percent defective? has...
-
What is the logical ending point of a sequential game that starts at position (2,8) with player 1 moving first? Show your work. Player 1 Strategy B Strategy A Strategy A Player 2 Strategy B (3,4)...
-
Problem A-6 Income and Retained Earnings Statements Peanut Corporation is a private corporation using ASPE. At December 31, 2017, an analysis of the accounts and discussions with company officials...
-
8.5 Area Between Curves (dy) Calculus-Calculator Allowed Mastery Check #2 Name: Date: Period: For 1-2, find the area of the region bounded by the following curves. Show the integral set up with...
-
Your company has a travel policy that reimburses employees for the "ordinary and necessary" costs of business travel. Employees often mix a business trip with pleasure by either extending the time at...
-
Simulation A: 1 Diameter 600 mm 2 Focal Length 1800 mm 3 F/D Ratio 3 4 Eyepieces 30 m 5 Barlow? N 6 Celestial Sights M42 - M31 - M51 Simulation B: 1 Diameter 150 mm 2 Focal Length 1800 mm 3 F/D Ratio...
-
The decision theoretic approach to set estimation can be quite useful (see Exercise 9.56) but it can also give some unsettling results, showing the need for thoughtful implementation. Consider again...
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
The concentration of Pb 2+ in a solution saturated with PbBr 2 (s) is 2.14 10 -2 M. Calculate K sp for PbBr 2 .
-
Approximately 0.14 g nickel(II) hydroxide, Ni(OH) 2 (s), dissolves per liter of water at 20 C. Calculate K sp for Ni(OH) 2 (s) at this temperature.
-
Write balanced equations for the dissolution reactions and the corresponding solubility product expressions for each of the following solids. a. Ag 2 CO 3 b. Ce(IO 3 ) 3 c. BaF 2
-
Manitoba ( Pty ) Ltd manufactures and sells Manita Herbal Teabags as a healthy product. The company is based in South Coast, KwaZulu - Natal, has a 3 1 December financial year end and applies...
-
1. An example of balance of payments accounting for Cascadial We will describe a series of transactions and assume that these are all the transactions that took place during the year so you can then...
-
A taxpayer may exclude which of the following from their gross income? An allocation of income from a business structured as a partnership, based on the taxpayer's percentage of ownership. An...

Study smarter with the SolutionInn App


