Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the
Question:
Classify each of the following reactions as a precipitation, acid–base, or gas-forming reaction. Show states for the products (s, ℓ, g, aq), and then balance the completed equation. Write the net ionic equation.
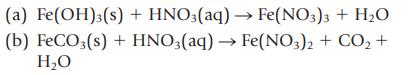
Transcribed Image Text:
(a) Fe(OH)3(s) + HNO3(aq) → Fe(NO3)3 + H₂O + HNO3(aq) → Fe(NO3)2 + CO₂ + (b) FeCO3(s) H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a FeOH3 s HNO3aq FeNO33aq H2O This is an acidbase reaction To balance the equation we need to ensure ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the products (s, , g, aq), and then balance the completed equation. Write the net ionic...
-
Classify each of the following reactions as one of the four possible types summarized in Table 19.3: (a) (b) (c) N2(g) 3 F2(g)2NF3(g) AH249 kJ; AS278 J/K N2(g) + 3C12(g) --> 2NC3(g) AH 460 kJ; AS...
-
Identify any one non governmental / non-profit organization in Toronto locality. Brief introduction to the organization that includes the following information: Vision/Mission Services / Programs...
-
Continuing with the preceding question, note that the monetary value of output in 1985 was$4,010billion in the United States and 1,418 billion cruzados in Brazil. Refer back to question 3 and...
-
The chain puller is used to draw two ends of a chain together in order to attach the master link. The device is operated by turning the screw S, which pushes the bar AB downward, thereby drawing the...
-
Why should companies use a projects free cash flows rather than accounting income when determining a projects NPV? AppendixLO1
-
The amounts of caffeine in a sample of five-ounce servings of brewed coffee are shown in the histogram. Make a frequency distribution for the data. Then use the table to estimate the sample mean and...
-
SULUI PUgram Question 6 - Week 12 (11 marks) Richard is a retired solicitor. His wife Tracy is a retired school teacher. Both wish to remain active and they invest in a gift shop that is to be...
-
Balance each of the following equations, and classify them as precipitation, acidbase, gas-forming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) CuCl + HS...
-
Give a formula for each of the following compounds: (a) A soluble compound containing the bromide ion (b) An insoluble hydroxide (c) An insoluble carbonate (d) A soluble nitrate-containing compound...
-
Evaluate Investment Choice and Its Impact on Performance Measures, with Joint Costs: Amberina, Inc., operates several different semiautonomous divisions. A problem arose with respect to two divisions...
-
A study was conducted to determine the proportion of people who dream in black and white instead of color. Among 296 people over the age of 55, 73 dream in black and white, and among 294 people under...
-
The other strategy could be to develop a completely distinct product line. This would allow Nike to develop sustainable products without affecting their main products. It could target specific green...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Cash Flows Horiz Analysis Horiz Analysis Vertic Analysis Vertic Analysis from Oper Inc St Bal St Inc St Bal Sheet Ratios Requirement Prepare the cash flows from operations section of R. Ashburn...
-
Transaction data and journal entries for McCall Real Estate Agency are presented in E3-8 and E3-9. Instructions (a) Post the transactions to T-accounts. (b) Prepare a trial balance at October 31,...
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
Consider the following two compounds. When treated with NaOH, one of these compounds forms an epoxide quite rapidly, while the other forms an epoxide very slowly. Identify which compound reacts more...
-
One mole of Ar initially at 310. K undergoes an adiabatic expansion against a pressure P external = 0 from a volume of 8.5 L to a volume of 82.0 L. Calculate the final temperature using the ideal gas...
-
Predict the products for each of the following reactions: a. b. c. d. . Ti[OCH(CH,),1. (+)-DET -- THCICH) (-)-DET
-
Prepare a 2017 T1 Return given the following information: Complete the T1 Income Tax Return and any relevant Schedules. Name: Mr. Henry Connor Age: 58 Years Old Province: Saskatchewan Gross Salary...
-
Date Aug 1 16 10 19 28 31 Activities Beginning inventory Purchase Sales Purchase Purchase Sales Units Acquired at Cost Units Sold at Retail 30 units @ $150 per unit 20 units @ $155 per unit 35 units...
-
Polaski Company manufactures and sells a single product called a Ret. Operating at capacity, the company can produce and sell 42,000 Rets per year. Costs associated with this level of production and...

Study smarter with the SolutionInn App


