Identify and name the water-insoluble product in each reaction and write the net ionic equation: (a) CuCl(aq)
Question:
Identify and name the water-insoluble product in each reaction and write the net ionic equation: 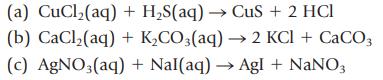
Transcribed Image Text:
(a) CuCl₂(aq) + H₂S(aq) → CuS + 2 HCI (b) CaCl₂(aq) + K₂CO3(aq) → 2 KCl + CaCO3 (c) AgNO3(aq) + Nal(aq) → Agl + NaNO3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To identify the waterinsoluble product in each reaction and write the net ionic equation you need to ...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Dracaris Inc. purchased an investment and expected to earn: Year 1 2 3 TV Net Cash Flows Php250 Php300 Php450 Php900 Assuming a 10% discount factor, how much is the net present value based on the...
-
1) Write balanced net ionic equation for the neutralization ofequal molar amounts of HNO2 and KOH 2) Write balanced net ionic equation for the neutralization ofequal molar amounts of HBr and NH3 3)...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
If the Federal Government increases taxes:What will be the effect on money demand, money supply, and interest rates? Money demand decreases, money supply is unchanged, and interest rates decrease...
-
In the late 1970s, Britain seemed to have struck it rich. Having developed its North Sea oil-producing fields in earlier years, Britain suddenly found its real income higher as a result of a dramatic...
-
Determine the moment of inertia for the parallelogram about the x' axis, which passes through the centroid C of the area. C
-
In Table 12.2, we assumed that output would not change if the old machine was replaced. Suppose output would actually double. How would this change be dealt with in the framework of Table 12.2?...
-
PRM Energy Systems, Inc. (PRM), owned technology patents that it licensed to Primenergy to use and to sublicense in the United States. The agreement stated that all disputes would be settled by...
-
Problem 13-69 (Algorithmic) (LO. 6) Katie exchanges a building and land (used in her business) for Tyler's land and building and some equipment (used in his business). The assets have the following...
-
Bromine is obtained from sea water by the following redox reaction (a) What has been oxidized? What has been reduced? (b) Identify the oxidizing and reducing agents. Cl(g) + 2 NaBr(aq) 2 NaCl(aq) +...
-
Name two anions that combine with Al 3+ ion to produce water-soluble compounds.
-
If the vehicle used as an example in this chapter accelerates to 50 km/h between each stop light, find the maximum distance between stoplights for which the energy used to accelerate the vehicle...
-
Newfoundland Hapset will be remitted to _____
-
The Z Energy Corp. has a new investment opportunity that generates cash flows of $6 million per year (in expectation) forever. The managers of Z Energy are not sure what the required rate of return...
-
Define an interface TwoStrings Oper declaring a function apply which takes two strings and returns a string. Then, define four classes implementing this interface, where the operation on strings...
-
Noeleen AutoMall, Ltd. recently completed an initial public offering(IPO) for$23,000,000 by listing its common shares on the New York Stock Exchange. Prior to itsIPO, Noeleen was a privately held...
-
Process Costing- increased units, FIFO method Answer in good form 25 26 Illustrative Problem-Cost of Production Report using Treatment by Neglect Dept 1-100% of materials are added at the beginning....
-
Review the transactions listed in E3-1 for Thyme Advertising Company. Classify each transaction as either an operating activity, investing activity, or financing activity, or if no cash is exchanged,...
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
Consider the oxidation of the amino acid glycine NH 2 CH 2 COOH to produce water, carbon dioxide, and urea NH 2 CONH 2 : NH 2 CH 2 COOH(s) + 3O 2 (g) NH 2 CONH 2 (s) + 3CO 2 (g) + 3H 2 O(l)...
-
Show what reagents you would use to prepare each of the following ethers via a Williamson ether synthesis, and explain your reasoning. a. b. c. OMe
-
Calculate the average CH bond enthalpy in methane using the data tables. Calculate the percent error in equating the average CH bond energy in Table 4.3 with the bond enthalpy. Table 4.3 Selected...
-
Managerial accounting provides information to internal users as they request it. This can be immediate and as frequent as demanded True or False True False
-
June Power Drive Corporation designs and produces a line of golf equipment and golf apparel. Power Drive has 100,000 shares of common stock outstanding as of the beginning of 2021. Power Drive has...
-
When the company follows the method of capitalizing borrowing costs, the steady income from investing temporarily surplus amounts of the borrowed amount is treated specifically for the construction...
Quality Assurance For Chemistry And Environmental Science 1st Edition - ISBN: 3642426441 - Free Book

Study smarter with the SolutionInn App


