Sulfanilic acid, which is used in making dyes, is made by reacting aniline with sulfuric acid. (a)
Question:
Sulfanilic acid, which is used in making dyes, is made by reacting aniline with sulfuric acid.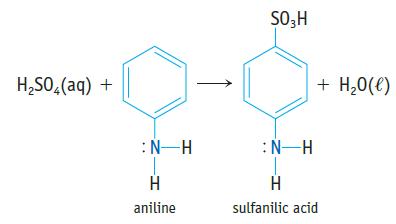
(a) Is aniline a Brønsted base, a Lewis base, or both? Explain, using its possible reactions with HCl, BF3, or other acid.
(b) Sulfanilic acid has a pKa value of 3.23. The sodium salt of the acid, Na(H2NC6H4SO3), is quite soluble in water. If you dissolve 1.25 g of the salt in water to give 125 mL of solution, what is the pH of the solution?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





