The enthalpy change for the oxidation of styrene, C 8 H 8 , is measured by calorimetry.
Question:
The enthalpy change for the oxidation of styrene, C8H8, is measured by calorimetry.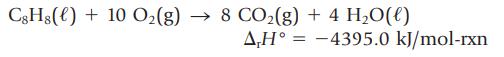
Use this value, along with the standard enthalpies of formation of CO2(g) and H2O(ℓ), to calculate the enthalpy of formation of styrene, in kJ/mol.
Transcribed Image Text:
C8Hg() + 10 O₂(g) → 8 CO₂(g) + 4H₂O(l) AH-4395.0 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To calculate the enthalpy of formation of styrene C8H8 i will use the enthalpy change for th...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
With reference to the "Chemistry Put to Work" box on explosives, (a) Use bond enthalpies to estimate the enthalpy change for the explosion of 1.00 g of nitroglycerin. (b) Write a balanced equation...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
2. Using the data below, create the project schedule using normal times. Determine the order in which you would crash the project one day, two days, and so on until it is in an all-crash mode....
-
Describe the three types of technologies. Explain the strategic role of technology.
-
A vaulted roof truss is loaded as shown. Determine the force in members HJ, IJ, and GI. STAN
-
Using risk-neutral valuation, derive a formula for a derivative that pays cash flows over the next two periods. Assume the risk-free rate is 4 percent per period. The underlying asset, which pays no...
-
Did Beaver have the contractual capacity to enter a contract with an exculpatory clause? Why or why not? Renee Beaver started racing go-karts competitively in 1997, when she was fourteen. Many of the...
-
At the beginning of its 2021 tax year, Hiram owned the following business assets: Date Placed in Service Initial Cost Accumulated Depreciation Recovery Period Depreciation Convention Furniture...
-
The following terms are used extensively in thermodynamics. Define each and give an example. (a) Exothermic and endothermic (b) System and surroundings (c) Specific heat capacity (d) State function...
-
The enthalpy change for the oxidation of naphthalene, C 10 H 8 , is measured by calorimetry. Use this value, along with the standard enthalpies of formation of CO 2 (g) and H 2 O(), to calculate the...
-
On a work sheet, where will the amount of the ending merchandise inventory be recorded?
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
Morgan Stanley is a leading investment bank founded in 1935. The company's fiscal year ends December 31, 2013, and it filed its financial statements with the SEC on February 25, 2014. On February 5,...
-
On 1 July 2018, Parent Ltd acquired all the shares of Son Ltd, on a cum-div. basis, for $2,057,000. At this date, the equity of Son Ltd consisted of: $ 1,000,000 Share capital 500 000 shares...
-
The vapor pressure of a liquid can be written in the empirical form known as the Antoine equation, where A(1), A(2), and A(3) are constants determined from measurements: Starting with this equation,...
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72 kJ mol -1 at 1 atm pressure. Calculate the normal and standard boiling points. Does your result for...
-
Pension data for Barry Financial Services Inc. include the following: ($ in thousands) $ 390 Discount rate, 7% Expected return on plan assets, 98 Actual return on plan assets, 8% Service cost, 2021...
-
On March 11, at the end of a pay period. Global Filter Corp's Payroll Register showed that its 23 empkypes had earned $21.000 of salos salaries and 54 root office salaries Withholdings from the...
-
3. Special Orders (1.5pts): Dog Man Donuts, Inc. makes and sells pre-packaged donuts. Each pre-packaged box of donuts regularly sells for $8.00 each. The firm typically produces and sells 1,600 boxes...

Study smarter with the SolutionInn App


