The following mechanism is proposed for a reaction: (a) Write the overall equation for the reaction. (b)
Question:
The following mechanism is proposed for a reaction:
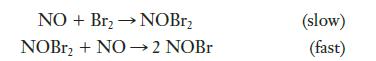
(a) Write the overall equation for the reaction.
(b) What is the rate-determining step?
(c) What is the intermediate in this reaction?
(d) What is the molecularity of each step of the reaction?
(e) Write the rate expression for each step.
Transcribed Image Text:
NO + Br₂ → NOBr₂ NOBr₂ + NO→ 2 NOBr (slow) (fast)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a 2 NO Br 2 2 NOBr b The firs...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The following mechanism is proposed for the reduction of NO3- by MoCl62-: a. What is the intermediate? b. Derive an expression for the rate law (rate = d[NO2-]/dt) for the overall reaction using the...
-
X died leaving the following properties: a. House and lot in the Philippines (mortgaged for P300,000) b. Condominium unit in Hongkong c. Car in Philippines d. Car in Hongkong e. Franchise exercised...
-
The decomposition of N 2 O 5 is given by the equation: The following mechanism is proposed for this reaction: (a) Does this mechanism as written provide the correct stoichiometry? If not, how does it...
-
Let a = (123) (45) = S, and b = (23) (14) = S5, then aba is equal to (13) (25) (135) (24) (15) (23) (123) (45)
-
Refer to Practice 188. Assume that the convertible preferred stock was issued on February 1. Also assume that the issuance agreement stipulates that the preferred stockholders are entitled to their...
-
Discuss the pros and cons of a retailer's developing new stores in domestic markets instead of China and India.
-
How can IBM communicate its strategy to companies, cities, and governments?
-
The post-closing trial balances of two proprietorships on January 1, 2010, are presented below. John and Calvin decide to form a partnership, John-Calvin Company, with the following agreed upon...
-
1. A taxpayer can go all the way to the U.S. Supreme Court to challenge the IRS in a tax disagreement. True False 2. The income tax system in the U.S. is a proportional tax system. True False
-
HBr is oxidized in the following reaction: (a) Show that this mechanism can account for the correct stoichiometry. (b) Identify all intermediates in this mechanism. (c) What is the molecularity of...
-
What are the reaction intermediates in the Chapman cycle?
-
A Brayton cycle inlet is at 300 K, 100 kPa and the combustion adds 670kJ/kg. The maximum temperature is 1200 K due to material considerations. What is the maximum allowed compression ratio? For this...
-
IV. Normal Distribution 9. IQ scores are said to be normally distributed with a mean of 100 and a standard deviation of 15. Label the normal curve below and then answer the questions that follow. a....
-
Manually determine the range, variance, and standard deviation of the set of numbers. Show the computations: a. 3, 8, 10, 14, 9, 10, 12, 21, 5, 13, 11, 10 b. 232, 212, 151, 325, 142, 132, 142, 236,...
-
Alex and Bess have been in partnership for many years. The partners, who share profits and losses on a 70:30 basis, respectively, wish to retire and have agreed to liquidate the business. Liquidation...
-
1 Frequency Domain Analysis 1. Given an input u(t) = cos(t) + 2 sin(5t) cos(5t) which is a sum of a lower frequency signal and a higher-frequency noise. Determine the feasible range of time constant...
-
A motor supplies a constant torque or twist of M = 120 lb ft to the drum. If the drum has a weight of 30 lb and a radius of gyration of ko = 0.8 ft, determine the speed of the 15-lb crate A after it...
-
Propose two syntheses of trnas-1-methyl-2-(methylthio)cyclohexane (shown in the margin), beginning with the starting compound (a) cis-1-chloro-2-methylcycIohexane; (b)...
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
a. For the hydrogenbromine reaction presented in Problem P36.7, imagine initiating the reaction with only Br 2 and H 2 present. Demonstrate that the rate law expression at t = 0 reduces to b. The...
-
The hydrogenbromine reaction corresponds to the production of HBr(g) from H 2 (g) and Br 2 (g) as follows: H 2 (g) + Br 2 (g) 2HBr(g). This reaction is famous for its complex rate law, determined by...
-
Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S²) through the following mechanism involving an intermediate helix (IH): a. Derive the rate law...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


