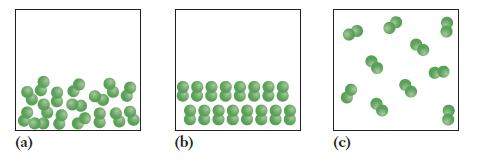
The molecular scale drawings below represent the three phases of iodine (I 2 ), which is a
Question:
The molecular scale drawings below represent the three phases of iodine (I2), which is a solid at normal temperature and pressure.
(a) In what order should the pictures be placed to put them in terms of decreasing entropy?
(b) Assuming the pressure is the same in all three cases, in what order should they be placed to put them in order of increasing temperature?
(c) Which picture depicts a situation in which enthalpy is the most important factor for the free energy, and in which picture is entropy the most important factor?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





