The picture shown depicts the species present at the start of a combustion reaction between methane, CH
Question:
The picture shown depicts the species present at the start of a combustion reaction between methane, CH4, and oxygen, O2.
(a) What is the limiting reactant?
(b) Draw the resulting state after this set of reactants has reacted as far as possible.
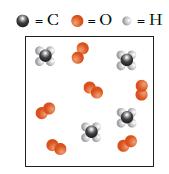
Transcribed Image Text:
= C = 0 = H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To determine the limiting reactant we need to consider the stoichiometry of the combustion reaction ...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The picture shown depicts the species present at the start of a combustion reaction between methane, CH 4 , and oxygen, O 2 . (a) Draw the resulting state after this set of reactants has reacted as...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Methane combustion on La2O3-based catalysts has been studied by Toops et al. [46]. With a 4% Sr-promoted La2O3 catalyst (2:5m2g-1) operating between 773 and 973 K, 0:5 -5 Torr CH4 and 3 _ 23 Torr O2...
-
1/ You just started working and you planned to save $5,000 every year in your retirement account. How much money will you have in your retirement account once you retire in 40 years? Your retirement...
-
Leonard Industries wishes to prepare a pro forma balance sheet for December 31, 2013. The firm expects 2013 sales to total $3,000,000. The following information has been gathered. (1) A minimum cash...
-
The biweekly taxable wages for the employees of Wee-Ones Foods follow. Compute the FICA taxes for each employee and the employer?s FICA taxes. FICA Taxes Biweekly Taxable Wages Employee No. Employee...
-
In the world of work, how do you end up writing a news release in the first place? Likely, the situation is this: your boss has handed you several pages of information and told you to write a news...
-
(Multiple Choice) 1. Reed Company had $375,000 of current assets and $150,000 of current liabilities before borrowing $75,000 from the bank with a 3-month note payable. What effect did the borrowing...
-
3IA Mccabe Corporation uses the weighted average method in its process costing. The following data pertain to its Assembly Department for September. Percent Complete Units Materials Conversion Work...
-
New chemistry students sometimes try to use the dilution equation, M 1 V 1 = M 2 V 2 to solve solution stoichiometry problems. (a) What concept is being overlooked by a student who takes this...
-
The particulate scale drawing shown depicts the products of a reaction between N 2 and O 2 molecules. (a) Draw a similar representation for the reactants that must have been present before the...
-
Let z be a random variable with a standard normal distribution. Find the indicated probability and shade the corresponding area under the standard normal curve. P( 0.82 z 0)
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
Let $N$ be a positive integer. Consider the relation $\circledast$ among pairs of integers $r, s \in \mathbb{Z}$ defined as $r \circledast s$ when $r-s$ is an integer multiple of $N$. Prove that...
-
Show that each summand in the differential equation for a Sturm-Liouville boundary value problem is self-adjoint. Then use the method of Exercise 7.5.21 to recover the self-adjoint form (11.155).
-
a. Determine the domain and range of the following functions.b. Graph each function using a graphing utility. Be sure to experiment with the window and orientation to give the best perspective of the...
-
Draw a plausible mechanism for each of the following transformations: (a) (b) (c) (d) Meo, OMe [H,SO4] excess MeOH -H20
-
Draw a plausible mechanism for each of the following reactions: (a) (b) [H,SO4] -H20 [H,SO4] -H20
-
Draw a plausible mechanism for each of the following reactions: (a) (b) HO HO- [H2SO4] -H20
-
4. Angela Bower's husband passed away last year. In 2019, Angela lives in a house with her son Jonathan (age 8) in Fairfield, Connecticut. She also supports her mother, Mona (age 67), who lives just...
-
What is the Net Present Value's assumption about how cash flows are re-invested? A, They are reinvested at the IRR. B, They are reinvested only at the end of the project. C, They are reinvested at...
-
Use the Excel file "PepsiCo Financial Statements" to answer the following questions. For full credit: a) You must show your work; and b) Your responses should be supported by empirical analysis (i.e....

Study smarter with the SolutionInn App


