A 1:1 mole ratio of CO (g) and H 2 (g) is called water gas.
Question:
A 1:1 mole ratio of CO (g) and H2 (g) is called water gas. It is used as a fuel because it can be burned in air: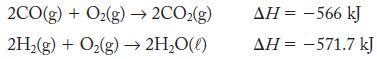
(a) Find the number of moles of CO (g) and H2 (g) Present in 10.0 g water gas. (Remember that they are present in a 1:1 mole ratio.)
(b) Use the preceding thermochemical equations to fi nd the enthalpy change when 10.0 g water gas is burned in air.
Transcribed Image Text:
2CO(g) +O₂(g)→ 2CO₂(g) 2H₂(g) + O₂(g) → 2H₂O(l) AH = -566 kJ AH = -571.7 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a To find the number of moles of CO g and H2 g present in 100 g of water gas we need to use their mo...View the full answer

Answered By

Rubina Kousar
I had done Msc Cs And doing a job at College Level I am very kind hearted to my students my method of teaching is verly good Not so strict My Teaching experience is very good Students are So obedient also i taught them in very pleasent environmnent.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
In a hydrogen fuel cell, hydrogen and oxygen gases react to produce water vapour. What volume of hydrogen at 40C and 150 kPa can be burned in a fuel cell using 300L of oxygen gas measured under the...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
9. A molybdenum-vanadium alloy of composition 50wt%Mo - 50wt%V is slowly cooled from a temperature of 2600C to 1800C. Determine: a) At what temperature does the first solid phase form? b) What is the...
-
The Kelvin equation, (17-16), predicts that solubility increases to infinity as the crystal diameter decreases to zero. However, measurements by L. Harbury [J. Phys. Chem., 50, 190-199 (1946)] for...
-
180% Declining balance for #12 and #13 Use the following to answer questions 5-15 (Straight Line, 180% declining balance and Activity Based) TTransport purchased a new semi-trailer truck for an...
-
What is the distinction between hedging and speculating!
-
Below is a project WBS with cost apportioned by percents. If the total project cost is estimated to be $600,000, what are the estimated costs for the following deliverables? a. Design? b....
-
New Homes has a bond issue with a coupon rate of 6.5 percent that matures in 16 years. The bonds have a par value of $1,000 and a market price of $1,022. Interest is paid semiannually. What is the...
-
Use the following data to calculate the variances in the following questions. Standard Cost Profile Lab Treatment SU #12 Expected Treatments= 1,000 Standard Cost Profile Lab Treatment SU #12 Expected...
-
What mass of ethylene, C 2 H 4 (g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is -1410.1 kJ/mol?
-
When a 2.30-g sample of magnesium dissolves in dilute hydrochloric acid, 16.25 kJ of heat is released. Determine the enthalpy change for the thermochemical equation Mg(s) + 2HCl(aq) MgCl(aq) + H2(g)...
-
Calculate, using the BlackScholes formula, the value of a call option given the following information: Interest rate = 7% Time to expiration = 90 days Stock price = $50 Exercise price = $45 ...
-
Time (s) Velocity (cm/s or m/s) Uncertainty 0.100 -145 cm/s or 0.145 m/s +/- 0.089 m/s 0.200 -266 cm/s or 0.266 m/s +/- 0.010 m/s 0.300 -359 cm/s or 0.359 m/s +/- 0.0201 m/s 0.400 -451 cm/s or 0.451...
-
Using Technology to Generate Normal Quantile Plots. In Exercises 13-16, use the data from the indicated exercise in this section. Use software (such as Statdisk, Minitab, Excel, or StatCrunch) or a...
-
Use your understanding of work and power to answer the following questions. 1. Two physics students, Will N. Andable and Ben Pumpiniron, are in the weightlifting room. Will lifts the 100-pound...
-
Problem 2. Consider the following chemical reaction. 2H2 + O2 = 2HO Gibbs Duhem equation states that SdT - Vdp+ Nidi=0. Apply this equation for the above reaction and determine the equilibrium...
-
Part D: Exploring Pascal's Triangle 1. Fill-In the missing numbers in Pascal's Triangle. See 2. Find the sum of each row in Pascal's Triangle. Describe the pattern. 1, 2, 4, 8, 16... Power of 2n 1 1...
-
Basic capital budgeting problem with straight-line depreciation. The Roberts Company has cash inflows of $140,000 per year on project A and cash outflows of $100,000 per year. The investment outlay...
-
Willingness to pay as a measure of a person's value for a particular good measures the maximum a person would be willing to pay requires that payment actually be made depends on the satisfaction that...
-
Describe what you would observe if you heated the liquid mixture at the composition corresponding to point i in Figure 9.24b from a temperature below T a to 118°C. Figure 9.24b 118 Vapor 100 Tj...
-
The heat of fusion of water is 6.008 10 3 J mol 1 at its normal melting point of 273.15 K. Calculate the freezing point depression constant K f .
-
At 39.9C, a solution of ethanol (x 1 = 0.9006, P * 1 = 130.4 Torr) and isooctane (P * 2 = 43.9 Torr) forms a vapor phase with y 1 = 0.6667 at a total pressure of 185.9 Torr. a. Calculate the activity...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


