A molecular model of hydrogen peroxide is shown below. Write the formula of hydrogen peroxide and use
Question:
A molecular model of hydrogen peroxide is shown below. Write the formula of hydrogen peroxide and use it to calculate the number of moles of hydrogen atoms in 0.011 mol hydrogen peroxide.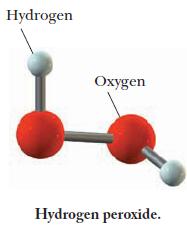
Transcribed Image Text:
Hydrogen Oxygen Hydrogen peroxide.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The formula of hydrogen peroxide is HO As you can see from the structural formula there are tw...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A molecular model of methyl alcohol is shown below; the OH group is an alcohol functional group, a group present in many important chemicals. Write the formula of methyl alcohol and use it to...
-
The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism: H2O2s(aq) + I (aq) H2O(I) + IO (aq) (slow) IO (aq) + H2O2(aq)...
-
Write a structural formula or build a molecular model of each of the following: (a) 1-Octyne (b) 2-Octyne (c) 3-Octyne (d) 4-Octyne (e) 2,5-Dimethyl-3-hexyne (f) 4-Ethyl-1-hexyne (g)...
-
According to the Statute of Frauds, in order to be legally enforceable, a contract must be in writing, name the contracting parties, identify the subject matter of the contract, and Be for a legal...
-
If the walls of the square waveguide in the previous problem are made of brass (ac = 1.5 107 S/m), find ac and the distance over which the wave is attenuated by 30%.
-
Which one of the following includes only financial budgets? i) cash budget and the operating budget ii) sales budget and the budgeted balance sheet iii) cash budget and the sales budget iiii)...
-
Personality traits and job performance. Refer to the Journal of Applied Psychology (January 2011) study of the determinants of task performance, Exercise 12.94 (p. 770). In addition to x1 =...
-
Andular Financial Services was organized on April 1 of the current year. On April 2, Andular prepaid $9,000 to the city for taxes (license fees) for the next 12 months and debited the prepaid taxes...
-
In 2023, Hiram, 15 and a dependent of his parents, had earned income of $514 and interest income of $8,193. Hiram's parents are in the 35% marginal tax bracket. Hiram has no deductible expenses. What...
-
On 1 June, Samuels & Co Advisors had balances in the Accounts Receivable and Allowance for Doubtful Debts accounts as set out below. Note transactions are GST Inclusive. 1/6 Balance b/d The following...
-
What is the percentage, by mass, of each element in the following substances? (a) C 4 H 8 (b) C 3 H 4 N 2 (c) Fe 2 O 3
-
Nickel tetracarbonyl, Ni(CO) 4 , is a volatile (easily converted to the gas phase), extremely toxic compound that forms when carbon monoxide gas is passed over finely divided nickel. Despite this...
-
World Class Rings produces class rings. Its best-selling model has a direct materials standard of 16 grams of a special alloy per ring. This special alloy has a standard cost of $63.30 per gram. In...
-
Problem 1 PROBLEMS Sabres Limited, a Canadian-controlled private corporation whose fiscal year end is December 31, provides you with the following data concerning its tax accounts and capital...
-
9.6. A habitual gambler often visits three different casinos and plays roulette there. He wants to discover at which casino he has better luck with his roulette games. So, he records his gambling...
-
The firm has estimated that its sales for 2 0 1 3 will be $ 8 4 6 , 7 5 6 Cash dividends to be paid by the firm in 2 0 1 3 $ 3 7 , 7 2 0 Minimum cash balance to be maintained by the firm $ 2 8 , 5 1...
-
Bob Long was hired by County Hospital aS supervisor of engineering and maintenance. Although well experienced in his field, this was his first management job. Soon after Bob's arrival a maintenance...
-
Initial Outlay (IO) 1. A company is considering purchasing a machine for $100,000. Shipping costs would be another $5,000. The project would require an initial investment in net working capital of...
-
Required: From the financial information below, complete an income statement, statement of changes in shareholders' equity, and balance sheet. Accounts receivable$ 4,000 Accounts payable 5,000 Cash...
-
1. Advertising for eyeglasses _________ (increases/decreases) the price of eyeglasses because advertising promotes _________. 2. An advertisement that succeeds in getting consumers to try the product...
-
Propose an efficient synthesis for the following transformation. Br
-
The mean solar flux at the Earths surface is ~2.00 J cm 2 min 1 . In a non-focusing solar collector, the temperature reaches a value of 79.5C. A heat engine is operated using the collector as the hot...
-
Propose a plausible mechanism for the following transformation. C CI
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


