At 2000 K, experiments show that the equilibrium constant for the formation of water is 1.6
Question:
At 2000 K, experiments show that the equilibrium constant for the formation of water is 1.6 × 1010.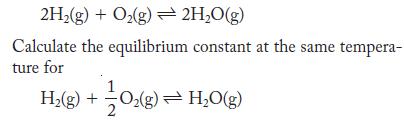
Transcribed Image Text:
2H(g) + O(g) 2HO(g) Calculate the equilibrium constant at the same tempera- ture for H(g) + O(g) = HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
K ...View the full answer

Answered By

Lalit Srivastava
I am a chemistry lover right from my graduation I am in love with chemistry I am reading and teaching chemistry from last 12 yrs and love to crack national level entrance exams . I cracked IIT Gate twice and CSIR Net once in 2018 held 28th general category.
I completed my graduation with 73 Percent and
M. sc with 69 percent of mark. I have been good in my academic but always loved the company of weak students. I love to know during class how to explain weak student.. And also having idea to make weaker student to understand and concept application.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for hydrate formation from acetaldehyde (CH3CHO) is 1, and at equilibrium, there is an equal amount of acetaldehyde and its hydrate (CH3CH(OH)2) in solution. The equilibrium...
-
Write sentences for the long-term direction and strategic path that management intends to follow. "Where we are headed?" and should explain why the direction in which you intend to point the company...
-
Show that the equilibrium is neutral in Prob. 10.2. Problem 10.2: Determine the vertical force P which must be applied at G so that the linkage is in equilibrium for the position shown. 20 b -5.5 in-...
-
can you complete this concept map that reviews some key ideas about species and speciation logy with Lab Connecting the Concepts: Species and Speciation oncepts: Species and Speciation oncept map...
-
What is the essence of the discounted cash flow methods?
-
1. Evaluate Nikes response to societal and consumer concerns about its contract manufacturing. 2. What are the challenges facing Nike in the future? Phil Knight and his University of Oregon track...
-
Does society have a moral right to interfere with individual behaviour in this way? 101-1
-
The Itsar Products Company has made the following monthly estimates of cash receipts and cash disbursements when preparing cash budgets for the next twelve months. Itsar Products has beginning cash...
-
14. The accounts of AB Partnership after its noncash assets were realized are as follows: Doble Credit Cash 34,000 Accounts payable 25,000 Loan payable to A 9,000 A Capital 8,000 B. Capital 8,000 In...
-
At 500 K, the equilibrium constant is 155 for H(g) + 1(g) 2HI(g) Calculate the equilibrium constant for HI(g) H(g) + (g) +1(g) =
-
An equilibrium mixture contains 3.00 mol CO, 2.00 mol Cl 2 , and 9.00 mol COCl 2 in a 50-L reaction flask at 800 K. Calculate the value of the equilibrium constant K c for the reaction at this...
-
Select the best answer for each of the following: 1. Johnson joined other creditors of Alpha Company in a composition agreement seeking to avoid the necessity of a bankruptcy proceeding against...
-
On Apple company with specific iPhone product Required to conduct a SWOT and PESTEL analysis, identifying the internal strengths and weaknesses and external opportunities and threats of the Apple...
-
In which social platforms are Walmart's brand/company active? In your opinion, are they doing a good job regarding customer engagement through social media channels? (Required: screenshots from the...
-
After you have watched both films, how would you describe each film? Also, consider what makes these early films different. List as many observations as you can that separate the Lumi re brothers...
-
How to develop the following points with the Poshmark application for second hand? 1. What are the main reasons for using this product? Or why not? 2. What are the hidden motivations? 3. Are there...
-
Suppose, in an experiment to determine the amount of sodium hypochlorite in bleach, you titrated a 22.84 mL sample of 0.0100 M K I O 3 with a solution of N a 2 S 2 O 3 of unknown concentration. The...
-
Write the condensed structural formula for each of the following compounds: (a) 2-ethyl-1-hexanol, (b) methyl phenyl ketone, (c) para-bromobenzoic acid, (d) ethyl butyl ether, (e) N,...
-
Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent,...
-
A bicycle is moving initially with a constant velocity along a level road. The bicyclist then decides to slow down, so she applies her brakes over a period of several seconds. Thereafter, she again...
-
Make a qualitative sketch of the position y as a function of time for the center of a yo-yo (the point at the middle of the axle). Also make sketches of the velocity and acceleration as functions of...
-
In SI units, velocity is measured in units of meters per second (m/s). Which of the following combinations of units can also be used to measure velocity? (a) Cm/s (b) Cm/s 2 (c) m 3 /(mm 2 s 2 ) (d)...
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


