At 500 K, the equilibrium constant is 155 for H(g) + 1(g) 2HI(g) Calculate the equilibrium constant
Question:
At 500 K, the equilibrium constant is 155 for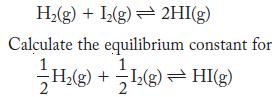
Transcribed Image Text:
H(g) + 1(g) 2HI(g) Calculate the equilibrium constant for HI(g) H(g) + (g) +1(g) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Solution HI Kc 15...View the full answer

Answered By

User l_960928
I completed my degree in 2017. I am working as an Accountant.But I can manage all the subjects and I am expert in that.I did tuitions to some of the childrens up to plustwo.So I have the experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
(a) At 800 K the equilibrium constant for I2(g) 2 I(g) is Kc = 3.1 Ã 10-5. If an equilibrium mixture in a 10.0-L vessel contains 2.67 Ã 10-2 g of I(g), how many grams of I2 are in the...
-
Consider the equilibrium dissociation of carbon dioxide CO 2 CO + 1/2 O 2 . At 2500 K, the equilibrium constant is 0.03635. Calculate the enthalpy of reaction for this reaction at 2500 K and use...
-
At 700 K the equilibrium constant for the reaction Is Kp= 0.76. A flask is charged with 2.00 atm of CCl4, which then reaches equilibrium at 700 K. (a) What fraction of the CCl4 is converted into C...
-
Determine the resultant force and specify where it acts on thebeam measured from A . Assume F = 540 lb . Part A Determine the magnitude of theresultant force. Part B Determine the distance between A...
-
Only quantitative outcomes are relevant in capital budgeting analysis. Do you agree? Explain.
-
What should be done to help ensure that Ponzi schemes like this one do not happen in the future? The fraud perpetrated by Bernard Madoff that was discovered in December 2008 was what is known as a...
-
Are you aware of having your own behaviour conditioned in this way? 101-1
-
SUPERVALU, one of the largest grocery retailers in the United States, is headquartered in Minneapolis. The following financial information (in millions) was taken from the companys 2010 annual...
-
I need the answer as soon as possible 28 On 1/1/2020, P Inc.acquired all of S Inc's common shares for cash equal to the stock's book value. The book value amounts of S assets and liabilities...
-
If 200 mL of 0.010 M CaCl 2 (aq) is mixed with 300 mL of 0.150 M NaOH(aq), will Ca(OH) 2 precipitate?
-
At 2000 K, experiments show that the equilibrium constant for the formation of water is 1.6 10 10 . 2H(g) + O(g) 2HO(g) Calculate the equilibrium constant at the same tempera- ture for H(g) + O(g) =...
-
A man (m 1 = 90 kg) is standing on a railroad flat car that is 9.0 m long and of unknown mass m 2 . The man and the car are initially at rest on a level track (Fig. P7.74A), and the wheels of the car...
-
1. Identify areas of difference that could potentially cause conflict between line managers in the United States and their employees who are natives of the locales in which they work (e.g. paid leave...
-
In organizational behavior, understanding the factors that predict organizational performance is crucial for managers and researchers. Numerous theories and studies have attempted to shed light on...
-
What emotional triggers or psychological biases influence consumer decision-making in our industry, and how can we leverage them in our marketing campaigns?
-
The most abundant protein on Earth, Rubisco, is a 530,000.0 g/mol enzyme involved in photosynthesis. You dissolve 33.568 g of Rubisco in 250 ml of water at 25.0 C. Assume the mixture behaves as an...
-
This week you studied about Organizational Development interventions. Proposed Intervention: Detail and explain the type of intervention (individual, team, or organizational) that is warranted for...
-
Draw the structure for 2-bromo-2-chloro-3-methylpentane, and indicate any chiral carbons in the molecule.
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
A person stands on level ground and throws a baseball straight upward, into the air. (a) Does the person exert a force on the ball while it is in his hand? After it leaves his hand? (b) Does the ball...
-
Figure P2.15 shows several hypothetical positiontime graphs. For each graph, sketch qualitatively the corresponding velocity time graph. Figure P2.15 Case 1 Case 2 Case 3
-
Figure Q2.15 shows a motion diagram for a rocket powered car. The photos are taken at 1.0-s intervals. Make qualitative plots of the position, velocity, acceleration, and force on the car as...
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...

Study smarter with the SolutionInn App


