Calculate H for Mg(s) + O(g) MgO(s) 2 AH = ? given the equations Mg(s) +
Question:
Calculate ΔH for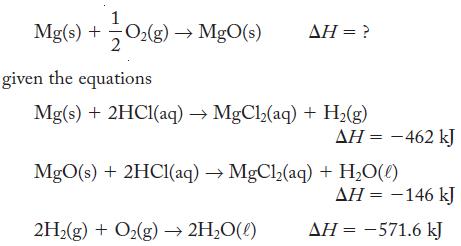
Transcribed Image Text:
Mg(s) + O₂(g) → MgO(s) 2 AH = ? given the equations Mg(s) + 2HCl(aq) →MgCl₂(aq) + H₂(g) 2H₂(g) + O₂(g) → 2H₂O(l) ΔΗ = -462 kJ MgO(s) + 2HC1(aq) → MgCl₂(aq) + H₂O(0) ΔΗ = -146 kJ AH = -571.6 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To find the enthalpy change AH for the reaction 1 Mgs O2g MgOs well use the given equations and perf...View the full answer

Answered By

Sumit kumar
Education details:
QUATERNARY Pursuing M.Tech.(2017-2019) in Electronics and Communication Engg. (VLSI DESIGN) from
GNIOT Greater Noida
TERTIARY B.Tech. (2012-2016) in Electronics and Communication Engg. from GLBITM Greater Noida
SECONDARY Senior Secondary School Examination (Class XII) in 2012 from R.S.S.Inter College, Noida
ELEMENTARY Secondary School Examination (Class X) in 2010 from New R.J.C. Public School ,Noida
CERTIFICATION
Summer Training in ‘WIRELESS EMBEDDED SYSTEM’ from ‘XIONEE’ for the six weeks.
EMBEDDED SYSTEM Certificate issued by CETPA INFOTECH for one day workshop.
Certificate of Faculty development program on OPTICAL COMMUNICATION and NETWORKS for one week.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
H Ltd has one subsidiary, S Ltd. The company has held a controlling interest for several years. The latest financial statements for the two companies and the consolidated financial statements for the...
-
Shareholders equity: Share capital VAN GOGH LIGHTING INC. Statement of Financial Position (partial) 0.7% 1.2% 2.0% 1.6% 2018 S8 non-cumulative preferred shares, redeemable, 50,000 shares authorized,...
-
From the work sheet, prepare the following: 1. Closing entries for Gimbel's Gifts and Gadgets in a general journal. 2. A post-closing trialbalance. Gimbel's Gifts and Gadgets Work Sheet For Year...
-
A 2.4-kg object is attached to a horizontal spring of force constant k = 4.5kN/m. The spring is stretched 10 cm from equilibrium and released. Find its total energy.
-
The inner and outer conductors of a coaxial cable have radii of 0.5 cm and 1 cm, respectively. The conductors are made of copper with r = 1, r = 1 and 5.8 x...
-
Particulate plc is an all-equity financed business with a market value of 35m and a cost of capital (after tax) of 20 per cent p.a. The business intends to purchase and cancel 8m of equity finance...
-
Edward Johnson III, the CEO and principal owner of the worlds largest mutual fund company, Fidelity Investments, Inc., was a longtime tennis buddy of Richard Larson. In 1995, Johnson asked Larson,...
-
Calculating and interpreting current yield and yield to maturity: Describe and differentiate between a bonds (a) current yield and (b) yield to maturity. Why are these yield measures important to the...
-
Professor Balkaran wants to sell his Bugatti Car and advertises it in the local FIU newspaper at $1,370,000, giving his telephone number, one of his students at FIU sees the advertisement and rings...
-
What are the molar concentrations of the ions in a 0.20 M calcium nitrate, Ca(NO 3 ) 2 , solution? Strategy In aqueous solution, Ca(NO 3 ) 2 dissociates into one Ca 2+ (aq) and two (aq) ions. Use the...
-
Describe the similarities and differences between the ways in which a gas and a liquid occupy a container.
-
Jivraj and Juma are accountants at Desktop Computers. Desktop Computers has not adopted the revaluation model for accounting for its property, plant, and equipment. The accountants disagree over the...
-
Indicate whether each of the following types of transactions will either (a) increase stockholders' equity or (b) decrease stockholders' equity: 1. expenses 2. revenues 3. stockholders' investments...
-
The following selected transactions were completed by Lindbergh Delivery Service during October: 1. Received cash from issuing capital stock, \($75,000\). 2. Paid rent for October, \($4,200\). 3....
-
Murray Kiser operates his own catering service. Summary financial data for February are presented in equation form as follows. Each line designated by a number indicates the effect of a transaction...
-
A. Given that y = e 2x + 1 complete the table of values of y corresponding to x = 0.5, 1 and 1.5. B. Use the trapezium rule, with all the values of y in the completed table, to obtain an estimate for...
-
Draw a schematic using NFETs and PFETs for a restoring logic gate that implements the function = 0 if zero or two of inputs cba are true. Assume that all inputs and their complements are available.
-
Two 30-cm-square vertical plates are separated by a distance of 2.5 cm and air at 1 atm. The two plates are maintained at temperatures of 200 and 90oC, respectively. Calculate the heat-transfer rate...
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
In explaining molecular structure, the MO model uses the change in MO energy with bond angle. Explain why the decrease in energy of the 1 a 1 and 2 a 1 MOs as 2 decreases more than offsets the...
-
The angular functions, Π(θ)Φ(), for the one-electron HartreeFock orbitals are the same as for the hydrogen atom, and the radial functions and radial probability...
-
What is the in-plane amplitude of the wave functions describing the Ï network in the conjugated molecules shown in Figures 24.18 and 24.19? a-2B a-B a+B a+B a+2B a-2B a+2B- m=3 m=4 m=5 m=6...
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


