Following are drawings of two isomers of C 6 H 4 Cl 2 (benzene rings are frequently
Question:
Following are drawings of two isomers of C6H4Cl2 (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at each vertex). Indicate whether each is polar or nonpolar. Explain your answer.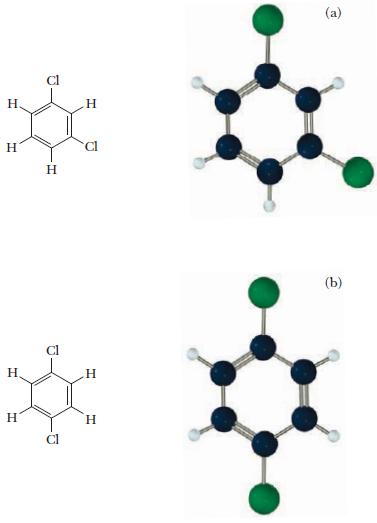
Transcribed Image Text:
H. H H H H 2 H Cl H H (a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The two isomers shown here are disubstituted benzene rings with two chlorine atoms attached Isomer a ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Capacity affects efficiency, speed, service and quality. Is there a right capacity for: A hospital? A hotel? A factory? A classroom? A university? Explain your answer
-
Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at...
-
Because the polarity of a molecule affects its physical properties, you need to know whether a molecule is polar or nonpolar when predicting how it might interact with other molecules. Predict...
-
Figure shows a cycle consisting of five paths: AB is isothermal at 300 K, BC is adiabatic with work = 5.0 J, CD is at a constant pressure of 5 atm, D E is isothermal, and EA is adiabatic with a...
-
Cash Flow what are some of the actions that a small company like The Grandmother Calendar Company can take if it finds itself in a situation in which growth in sales outstrips production capacity and...
-
Phoenix-based CompTronics manufactures audio speakers for desktop computers. The following data relate to the period just ended when the company produced and sold 42,000 speaker sets:...
-
Effectiveness of online and face-to-face learning. A study published in the e-Journal of Business Education & Scholarship of Teaching (Vol. 12, 2018) compares the effectiveness of online learning...
-
Assume that Lee agrees to the assignment of the house-painting contract to Karen as stated in question 3. Thereafter, Lee fails to perform the contract to paint Karens house. Karen sues Sally for...
-
The unadjusted trial balance of the Photo Sense Company as of December 31 is found on the trial balance tab. The following information is required to prepare the necessary adjusting entries for the...
-
Following are drawings of two derivatives of acetylene. Indicate whether each is polar or nonpolar, and explain your answer. (a) F-C=C-F (b) H-C=C-F
-
Identify the hybrid orbitals on the central atom that form the bonds in the following species. (a) CF 4 (b) SbCl 6 (c) AsF 5 (d) SiH 4 (e) NH 4
-
Should preferred stock be considered as equity or debt? Explain.
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
(a) According to Equation 24-2, if all conditions are constant, but particle size is reduced from 3 m to 0.7 m, by what factor must pressure be increased to maintain constant linear velocity? (b) If...
-
Juarez worked for Westarz Homes at construction sites for five years. Bever was a superintendent at construction sites, supervising subcontractors and moving trash from sites to landfills. He...
-
It is intended to manufacture a circular bar to resist torque; however, the bar is made elliptical in the process of manufacturing, with one dimension smaller than the other by a factor k as shown....
-
If a = 25 mm and b = 15 mm, determine the maximum shear stress in the circular and elliptical shafts when the applied torque is T = 80 N m. By what percentage is the shaft of circular cross section...
-
The brass wire has a triangular cross section, 2 mm on a side. If the yield stress for brass is t Y = 205 MPa, determine the maximum torque T to which it can be subjected so that the wire will not...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


