Given the thermochemical equations 2Cu(s) + Cl(g) 2CuCl(s) 2CuCl(s) + Cl(g) 2CuCl2(s) find the enthalpy change
Question:
Given the thermochemical equations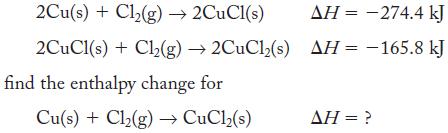
Transcribed Image Text:
2Cu(s) + Cl₂(g) → 2CuCl(s) 2CuCl(s) + Cl₂(g) →2CuCl2(s) find the enthalpy change for Cu(s) + Cl₂(g) → CuCl₂(s) AH = -274.4 kJ AH = -165.8 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
From the following data, calculate : (a) P/V Ratio. (b) Profit when sales are Rs. 40,000. (c) New break-even point if selling price is reduced by 20%. Fixed Expenses Rs. 8,000. Break-Even point Rs....
-
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO 2 they produce. The greater the heat relative to the...
-
Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following thermochemical equations CH4(g) + 20(g) CO(g) + 2HO(l) CH4(g) + 30(g) 2CO(g) + 2HO(l)...
-
A strain of E-coli Beu 397-recA441 is placed into a nutrient broth at 30° Celsius and allowed to grow. The data shown in the table are collected. The population is measured in grams and the time...
-
For the shrinking-core model, if the rate of leaching is controlled by an interface chemical reaction that is first order in the concentration of reactant A, derive the expression, where k =...
-
Garden Depot is a retailer that is preparing its budget for the upcoming fiscal year. Management has prepared the following summary of its budgeted cash flows: Total cash receipts Total cash...
-
What is the most widely used of all accounting statistics?
-
As financial manager of Britwell Inc., you are investigating a possible acquisition of Salome. You have the basic data given in the following table. You estimate that investors expect a steady growth...
-
3 points Financial information is presented below: The amount of net sales on the income statement would be: $355,500 $169,500 $134,500 $284,500
-
The three forces are applied to the bracket. Determine the range of values for the magnitude of force P so that the resultant of the three forces does not exceed 2400 N. 3000 N 90%- Prob. 2-38 800 N...
-
Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.71. Exercise 5.71 Given the thermochemical equations 2Cu(s) + Cl(g) 2CuCl(s) 2CuCl(s) + Cl(g) 2CuCl2(s) find the...
-
In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO(g) = -24.8 kJ The enthalpy change...
-
Nathvile Laundry reported assets of $800 and equity or $480. What is Nathvile's debt ratio? a.60% a.40% a.67% d. Not enough information is provided
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
Consider each of the following scenarios and identify a behavioral intervention to address each issue in family work. A teenager not complying with curfew. One member of the couple not picking up...
-
Sandy Crane Hospital expanded its maternity ward to add patient rooms for extended hospital stays. They negotiated a 15-year loan with monthly payments and a large sum of $250,000 due at the end of...
-
2 (39 marks) R QUESTION 2 (39 marks) Roundworm Ltd is a group of companies with a 31 December year-end. The Roundworm group financial statements for the years 20.21 and 20.22 are given below:...
-
Vino Veritas Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,400 cases of wine at a price of 240 euros per case. The total purchase price is 336,000...
-
Repeat Exercise 21.14 adding a check in T 1 so that Y does not exceed 90. In exercise 21.14 Change transaction T 2 in Figure 21.2b to read: read_item(X); X:= X+M; if X > 90 then exit else...
-
Conduct a VRIO analysis by ranking Husson University (in Maine) business school in terms of the following six dimensions relative to the top three rival schools. If you were the dean with a limited...
-
Why is the inequality < 1 always satisfied in dilute electrolyte solutions?
-
Under what conditions does 1 for electrolyte solutions?
-
How do you expect S m for an ion in solution to change as the ionic radius increases at constant charge?
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


