In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described
Question:
In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: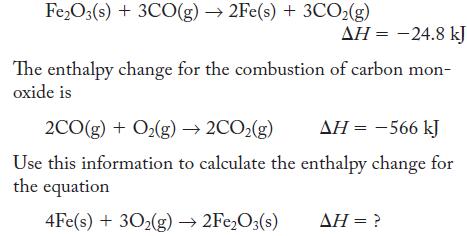
Transcribed Image Text:
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) ΔΗ = -24.8 kJ The enthalpy change for the combustion of carbon mon- oxide is 2CO(g) + O₂(g) → 2CO₂(g) AH = -566 kJ Use this information to calculate the enthalpy change for the equation 4Fe(s) + 302(g) → 2Fe₂O3(s) AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
The balanced chemical equation for the reaction of carbon monoxide CO ...View the full answer

Answered By

Mohiddin Shaik
I have Bachelors degree in Electrical and Electrons engineering
Masters degree in Control systems
I have 3 years experience in Teaching
I have 5 years years experience as Q&A expert.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What does an energy-level diagram for the reverse reaction from Exercise 5.72 look like? Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
Draw an energy-level diagram that represents the Hesss law calculation in Exercise 5.72. Exercise 5.72 In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as...
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
How many gallons of mercury (sg = 13.54) would weigh the same as 5 gal of castor oil, which has a specific weight of 59.69 lb/ft 3 ?
-
Repeat Example 16.8, except that the ore contains 3 wt% Cu 2 O
-
Please help me to answer possible answers to these questions below. I just need to know a general idea how the answer to these questions suppose to be. There is no word limit for each answer. Thank...
-
What is the normal balance of the Treasury Stock account?
-
A client, Shirlee James, requires 10 partner labor hours and 25 professional associate hours from O'Reilly and Shriberg, the law firm in E9-6. The partners of O'Reilly and Shriberg, attorneys-at-law,...
-
Stacy purchased an annuity contract for $21000. She begins to receive $3000 per year in 2017 for 21 years. What portion of the $3000 received is included in Stacey's gross and calm in the current...
-
Crane Library, a nonprofit organization, presented the following statement of financial position and statement of activities for its fiscal year ended February 28, 2024. Assets Current Assets Cash...
-
Given the thermochemical equations 2Cu(s) + Cl(g) 2CuCl(s) 2CuCl(s) + Cl(g) 2CuCl2(s) find the enthalpy change for Cu(s) + Cl(g) CuCl(s) AH = -274.4 kJ AH = -165.8 kJ AH = ?
-
Calculate H for the reaction Zn(s) + --0(g) ZnO(s) 2 given the equations AH = ? Zn(s) + 2HC1(aq) ZnCl (aq) + H(g) 2H(g) + O(g) 2HO(l) = -152.4 kJ ZnO(s) + 2HC1(aq) ZnCl(aq) + HO(0) AH = -90.2 kJ...
-
In Figure a, particle P is to move parallel to the x and x axes of reference frames S and S', at a certain velocity relative to frame S. Frame S' is to move parallel to the r axis of frame S at...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Payroll Payroll Register Register Thomas Avery Towle...
-
Name: Course: Worksheet Lab Experience 5 Logic Circuits (A) Exercise 5.1 Truth table for the example circuit A B Output Value 0 0 1 1 0 1 1 Exercise 5.2 A slight change in the example circuit...
-
Stanley Medical Hospital is a non-profit and a non-chartered hospital planning to acquire several hospitals in the area. The hospital is researching financial options since they want to expand into...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2022, for $12,000. They expect to use the...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
What implications would a no-steal/force buffer management policy have on checkpointing and recovery?
-
Trade credit from suppliers is a very costly source of funds when discounts are lost. Explain why many firms rely on this source of funds to finance their temporary working capital.
-
How do you expect S m for an ion in solution to change as the charge increases at constant ionic radius?
-
It takes considerable energy to dissociate NaCl in the gas phase. Why does this process occur spontaneously in an aqueous solution? Why does it not occur spontaneously in CCl 4 ?
-
Calculate S R for the reaction Ba(NO 3 ) 2 (aq) + 2KCl(aq) BaCl 2 (s) + 2KNO 3 (aq).
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


