Propene, C 3 H 6 , is proposed as a starting material in the production of butyraldehyde,
Question:
Propene, C3H6, is proposed as a starting material in the production of butyraldehyde, C4H8O.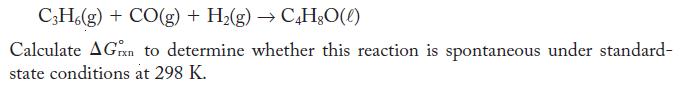
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
AG448 k...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Regression Statistics Multiple R R Square Adjusted R Square 0.8355 0.6980 0.6774 Standard Error 38.2611 Observations 48 ANOVA df SS MS F Significance F Regression 3 148889.8565 49629.9522 33.9023...
-
Year Project A (upgrade existing B-Wings) Project B (develop new X-Wings) 0 -$250,000 -$400,000 1 $100,000 $50,000 2 $80,000 $70,000 3 $60,000 $80,000 4 $40,000 $120,000 5 $20,000 $200,000 The above...
-
Succinic acid, which has the structure HOOCCH 2 CH 2 COOH and on a C-mole basis is represented by CH 3/2 O, is useful as a starting material in the manufacture of solvents and polymers. It is...
-
The Dulac Box plant produces 500 cypress packing boxes in two 10-hour shifts. What is the productivity of the plant?
-
The step-growth polymer nylon 6 is prepared from caprolactam. The reaction involves initial reaction of caprolactam with water to give an intermediate open?chain amino acid, followed by healing to...
-
a. Is it possible to process one applicant every minute? Explain. b. How would you assign tasks to workers in order to process 60 applicants an hour? c. How many workers are required? How efficient...
-
Does the organization have an appropriate amount of capacity?
-
Suppose that the government of Brazil took possession of the cacao farms of a chocolate factory owned by a U. S. firm. What rights would the U. S. factory have? What limits exist on those rights?
-
De la informacin provista a continuacin, el margen de utilidad neta es: Ventas = $1,500,000 Beneficio bruto 450.000 Ganancia neta antes de intereses e impuestos = $150,000 Utilidad neta despus de...
-
Calculate G at 25 C and at 300 C for the synthesis of ammonia under standard conditions to determine whether the reaction is spontaneous at those temperatures.
-
Calculate the standard entropy change for the decomposition of hydrogen peroxide into water and oxygen. The reaction is:
-
In Problem use the transition matrix to find S 1 and S 2 for the indicated initial state matrix S 0 . S 0 = [0 0 1] A .2 .4 4 P = B .7 .2 .1 .5 .3 .2
-
The Intel Outsourcing case case explores the make-versus-buy decision for the well-known chip maker. Use The Strategic Sourcing framework to examine this important decision for Intel. Use the...
-
Final-year students enrolled in the Interactive Multimedia course at Edith Cowan University are required to develop skills and expertise in managing the design and development of client websites. The...
-
Find the minimum tractive effort required for vehicle to maintain 70mph speed at 5%upgrade through an air density of 0.002045 slug/ft^3. Show all steps and unit conversion please Problem 2:...
-
Sanburn writes about the conflict of decreasing funding and enrollment for community colleges and the increasing value of an associate degree. Explain how those two factors can co - exist at the same...
-
Rare beauty new Shampoo and Conditioner Branding Strategy What is the branding strategy for your organization? What is the purpose of your brand? How will you differentiate yourself from domestic...
-
One of the emission lines of the hydrogen atom has a wavelength of 93.8 nm. (a) In what region of the electromagnetic spectrum is this emission found? (b) Determine the initial and final values of n...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Obtain the output v o in the circuit of Fig. 5.94 . 80 k2 80 k2 20 k2 40 k2 www ww- 1.2 V 20 k2 +. 2.8 V +,
-
Design a problem to help other students better understand how capacitors work. In 5 s, the voltage across a 40-mF capacitor changes from 160 V to 220 V. Calculate the average current through the...
-
Find v o in the op amp circuit of Fig. 5.65 . 8 v2 16 V1 +, 12 7.5 V 24 +)
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


