The equilibrium constant for the following reaction is 1.0 10 14 at 298 K . Strategy
Question:
The equilibrium constant for the following reaction is 1.0 × 1014 at 298 K .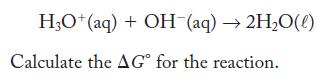
Strategy
We know that ΔG ° = -RT ln Keq, and we have values for Keq and T. Use the proper value and units for R so the units of ΔG ° are joules or kilojoules.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Using R 8314 JK and T 298 K we subst...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
List two similarities and two differences between the Safety Analysis of the Incident Algorithm and the BowTie Diagram. a. Example 6-1: Gas-Phase Reaction in a Microreactor Wolfram and Python 1. Use...
-
The equilibrium constant for dissociation of N2O4 is 0.664 and 0.141 at 318 K and 298 K respectively. Calculate the average heat of reaction within this temperature range.
-
How does science affect the selection process? Explain your reasoning
-
Butacetin is an analgesic (pain, killing) agent that is synthesized commercially from p-fluoronitrobenzene. Propose asynthesis. NHCOCH3 Butacetin (CH3)3CO
-
Prior to the invention of the printing press in the mid- 1400s, the process of producing fine books, called illuminated manuscripts, was strictly manual and performed by skilled craftsmen. A scribe...
-
What is the organization's market share, and has it been increasing or decreasing?
-
A contract calls for annual payments of $1,200. Find the present value of the contract, assuming that (1) The number of payments is 7 and the current interest rate is 6 percent: (2) The number of...
-
On January 1 , 2 0 X 1 , Diaz Corp. acquired all of the outstanding stock of Guled, Inc. for $ 6 , 0 0 0 , 0 0 0 . Guled, Inc. will continue to operate as a separate legal entity with independent...
-
The standard Gibbs free-energy change for the following reaction is +55.69 kJ . Strategy Because we know the G and the temperature, we can use Equation 17.11 to determine the equilibrium constant....
-
Calculate the Gibbs free-energy change for the reaction of nitrogen monoxide and bromine to form nitrosyl bromide at 298 K under two sets of conditions. (a) The partial pressure of each gas is 1.0...
-
A recent Apple iPad has a resolution of 2048 1536 and a diagonal scan size of 9.7 inches. What is the picture ratio of this display? What is the pixel density?
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
Molybdenum metal must absorb radiation with a minimum frequency of 1.09 1015s-1 before it can eject an electron from its surface via the photoelectric effect. (a) What is the minimum energy needed...
-
Suppose that the laptop of Prob. 2.16 is placed in an insulating briefcase with a fully charged battery, but it does not go into sleep mode, and the battery discharges as if the laptop were in use....
-
If the accuracy of positioning the probe described in Problem 9.5 is plus or minus 5.0 mm, compute the possible error in measuring the average velocity.
-
A small velocity probe is to be inserted through a pipe wall. If we measure from the outside of the DN 150 Schedule 80 pipe, how far (in mm) should the probe be inserted to sense the average velocity...
-
Compute points on the velocity profile from the tube wall to the centerline of a standard hydraulic steel tube, 50 mm OD 1.5 mm wall, if the volume flow rate of SAE 30 oil (sg = 0.89) at 110C is 25...
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


