The following data were obtained for the decomposition of nitrogen dioxide. 2NO2(g) 2NO(g) + O(g) Time (s)
Question:
The following data were obtained for the decomposition of nitrogen dioxide.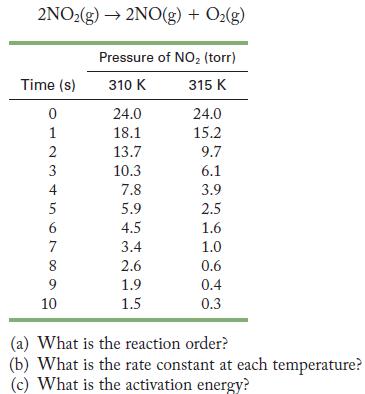
Transcribed Image Text:
2NO2(g) 2NO(g) + O(g) Time (s) 0 1 23456 4 7 8 9 10 Pressure of NO (torr) 310 K 315 K 24.0 18.1 13.7 10.3 7.8 5.9 4.5 3.4 2.6 1.9 1.5 24.0 15.2 9.7 6.1 3.9 2.5 1.6 1.0 0.6 0.4 0.3 (a) What is the reaction order? (b) What is the rate constant at each temperature? (c) What is the activation energy?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To determine the reaction order rate constant and activation energy we can use the integrated rate l...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Choose a logo from a women's professional sports team and a men's professional sports team and complete the following analysis: A1) Paste in the logo for your chosen women's pro sports team and...
-
The following values of the rate constant were obtained for the decomposition of nitrogen dioxide at various temperatures. Plot the logarithm of k versus 1/T and from the graph obtain the energy of...
-
The decomposition of nitrogen dioxide (NO2) into nitrogen monoxide (NO) and oxygen is a second-order reaction. This means that the concentration C of NO2 at time t is given by 1/C = kt + 1/C0, where...
-
The Electronic Industries Association reports that about 50% of U.S. households have a camcorder. For a randomly selected sample of 800 U.S. households, use the normal approximation to the binomial...
-
Find Vx in the circuit infigure. 24 V Vx 4 kn 36 kn 6V(+ +)8 V
-
Leonard Industries wishes to prepare a pro forma balance sheet for December 31, 2013. The firm expects 2013 sales to total $3,000,000. The following information has been gathered. (1) A minimum cash...
-
Am I prepared to put away my cell phone (and keep it turned off) or personal digital assistant during the entire interview? LO.1
-
On January 1, 2017, Hunter Ltd. entered into an agreement to lease a truck from Situ Ltd. Both Hunter and Situ use IFRS 16. The details of the agreement are as follows: Additional information: 1....
-
Given the following information find the company's beta. risk free rate 5% market risk premium 8% required return by investors 15% Options are as follows: .625, 1.00, 1.25, 0.77, 1.60
-
The reactant in a first-order reaction decreases in concentration from 0.451 to 0.235 M in 131 seconds. How long does it take to decrease from 0.235 to 0.100 M?
-
Use the following experimental data to determine the rate law and rate constant for formation of phosgene. CO + Cl COC1 Experiment 123 Initial Concentration (M) [CO] 0.053 0.106 0.106 [C] 0.23 0.23...
-
An American firm purchases \($4,000\) worth of perfume (Mex\($20,000)\) from a Mexican firm. The American distributor must make the payment in 90 days, in Mexican pesos. The following quotations and...
-
Briefly explain the difference between a k-factor model and the capital asset pricing model
-
Refer to the cost data, Picture below. Take off the square feet of wall forms and cubic yards of ready mix concrete for the walls of the elevator pit. Determine the total material and labor cost for...
-
possible Submit quiz A researcher studies water clarity at the same location in a lake on the same dates during the course of a year and repeats the measurements on the same dates 5 years later. The...
-
A liquid hydrocarbon mixture was made by adding 295 kg of benzene, 289 kg of toluene and 287 kg of p-xylene. Assume there is no change of volume upon mixing, i.e., Vmix=0 , in order to determine: 1....
-
b) Maseru Development Bank has R850 million credit with Matsieng Hydroelectric Power, with a maturity of eighteen months. The expected loss for Maseru Development Bank is R22 million, and the...
-
Which of the following molecules would you expect to be aromatic? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) N+
-
Explain why each of the following is either a private good or a public good: traffic lights, in line skates, a city park, a chicken salad sandwich, a tennis racket, national defense, a coastal...
-
Determine the slope and displacement at point B. EI is constant. Using Castiglianos theorem. 400 N 300 N/m 'A 3 m
-
Determine the slope and displacement at point B. EI is constant. Use the method of virtual work. 400 N 300 N/m 'A 3 m
-
Determine the displacement and slope at point C of the cantilever beam. The moment of inertia of each segment is indicated in the figure. Take E = 29(10 3 ). Using Castiglianos theorem. A IBc = 200...
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


