The most intense peak in a mass spectrum is assigned a height of 100 units. The following
Question:
The most intense peak in a mass spectrum is assigned a height of 100 units. The following spectrum was obtained from a sample of an element. Use the data to calculate the atomic mass of the element. Identify the element.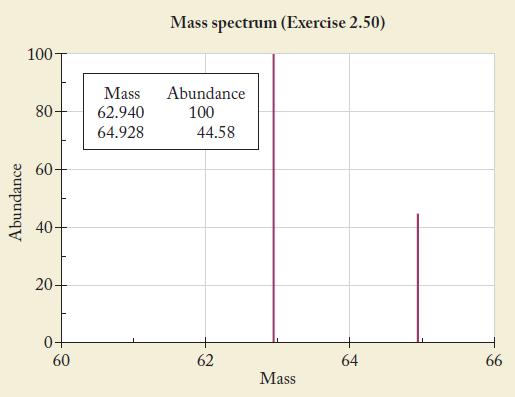
Transcribed Image Text:
Abundance 100- 80- 60 40 20- 60 Mass 62.940 64.928 Mass spectrum (Exercise 2.50) Abundance 100 44.58 62 Mass 64 66
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the average atomic mass of the element from the mass spectrum you need to consider both ...View the full answer

Answered By

Shivaji Thombare
I am Mr. Shivaji Thombare.
*Qualification*
M.Sc. Mathematics
(Savitribai Phule Pune University, Pune,India
B.Ed(Bachelor's degree in education)
(Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.)
*Experience*
I have worked as a Lecturer in Mathematics for 6 years in Jamkhed Mahavidyalaya,Jamkhed,India.I also tutor at Chegg and helped students all over world to solve mathematics problems for 2 Years. I am an easy-going person and see Mathematics as fun. I would like to help students in Mathematics. I love to teach students and see them succeed in life.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The most intense peak in a mass spectrum is assigned a height of 100 units. The following spectrum was obtained from a sample of an element. Use the data to calculate the atomic mass of the element....
-
The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic of Pb. The most intense peak in each envelope appears at m/z 326.0, 267.0, 224.0 and...
-
Polystyrene is a synthetic polymer with the structure - (CH2- CH (C6Hs)), A batch of polydisperse polystyrene was prepared by initiating the polymerization with t-butyl radicals. As a result, the...
-
The following is the stockholders' equity section from Chipotle Mexican Grill, Inc.'s balance sheet (in thousands, except per share data). a. Show the computation to derive the $375 thousand for...
-
A waveguide with lossless dielectric inside and perfectly conducting walls has a cross-sectional contour ? that departs slightly from a comparison contour Co whose fields are known. The difference in...
-
a. Design an observer for the dc-dc converter of Problem 36. The observer should have time constants 10 times smaller than those of the original system. b. Simulate your system and observer for a...
-
Let M be an SDF process and Y a labor income process. Assume E T 0 Mt|Yt|dt < for each finite T. The intertemporal budget constraint is dW = rW dt + ( r)dt +Y dt Cdt + dB. (14.34) (a) Suppose...
-
The astronaut orbiting the Earth in Figure P4.32 is preparing to dock with a Westar VI satellite. The satellite is in a circular orbit 600 km above the Earths surface, where the free-fall...
-
Prepare the income statement for South Marine Company for the most recent year. Use the calculation of cost of goods sold, cost of goods manufactured, and the amounts below. Assume that the company...
-
Antimony occurs naturally as two isotopes, one with a mass of 120.904 u and the other with a mass of 122.904 u. (a) Give the symbol that identifies each of these isotopes of antimony. (b) Get the...
-
The mass spectrum of an element shows that 92.2% of the atoms have a mass of 27.977 u, 4.67% have a mass of 28.976 u, and the remaining 3.10% have a mass of 29.974 u. (a) Calculate the atomic mass...
-
Brian Smith, network administrator at Advanced Energy Technology (AET), was given the responsibility of implementing the migration of a large data center to a new office location. Careful planning...
-
Safeway, Inc., operated 1,739 stores as of January 3, 2009. The following data were taken from the company's annual report. All dollar amounts are in thousands. Required a. Compute Safeway's...
-
Rich French, the owner of Rich's Fishing Supplies, is surprised at the amount of actual inventory at the end of the year. He thought there should be more inventory on hand based on the amount of...
-
Carol Lapaz owned a small company that sold boating equipment. The equipment was expensive, and a perpetual system was maintained for control purposes. Even so, lost, damaged, and stolen merchandise...
-
The following footnote related to accounting for inventory was taken from the 2008 annual report of Wal-Mart, Inc. Inventories The Company values inventories at the lower of cost or market as...
-
Plot the magnitude and phase of the frequency response of normalized n-th order lowpass Butterworth filters.
-
Isopropanol is prepared by reacting propylene (CH3CHCH2) with sulfuric acid, followed by treatment with water. (a) Show the sequence of steps leading to the product. What is the role of sulfuric...
-
What is an insurable interest? Why is it important?
-
An alkyne with molecular formula C 4 H 6 was treated with ozone followed by water to produce a carboxylic acid and carbon dioxide. Draw the expected product when the alkyne is treated with aqueous...
-
Starting with acetylene, show the reagents you would use to prepare the following compounds: (a) 1-Butyne (b) 2-Butyne (c) 3-Hexyne (d) 2-Hexyne (e) 1-Hexyne (f) 2-Heptyne (g) 3-Heptyne (h) 2-Octyne...
-
Preparation of 2,2-dimethyl-3-octyne cannot be achieved via alkylation of acetylene. Explain.
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


