Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride?
Appendix F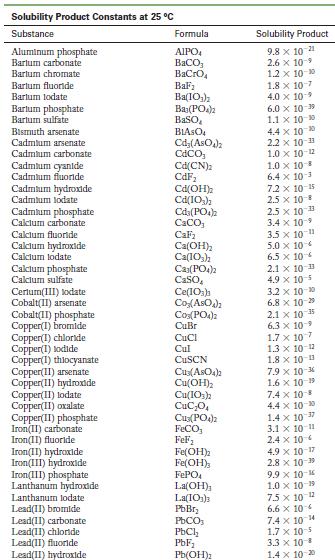

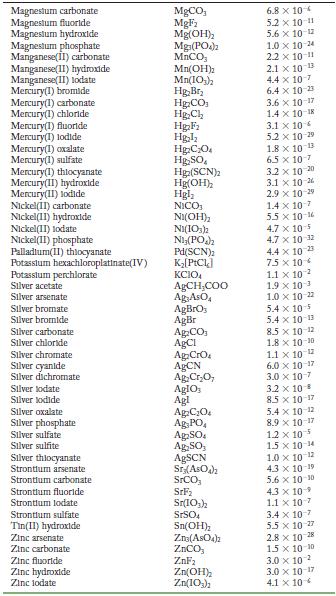
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper(1) chloride Copper(1) todide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II)oxalate Copper(II) phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(II) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Ba(PO4)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(103)2 Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103) Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cu3(ASO4)2 Cu(OH) Cu(103)2 CuC0 Cuz(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-36 1.6 x 10 19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
If we add 250 mL of 0010 M NaOH to 750 mL of a 010 M solution of magnesium chloride will a precipita...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 10 -6 M iron(II) chloride is added to 20.0 mL of 3.0 10 -4 M barium hydroxide....
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 10 -6 M magnesium chloride is added to 80.0 mL of 1.0 10 -6 M potassium fluoride....
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO 3 is added to 10 mL 0.0010 M Na 2 SO 4 . Appendix F Solubility Product...
-
On January 15, Tundra Co. sold merchandise to customers for cash of $42,000 (cost $28,500). Merchandise costing $10,500 was sold to customers for $15,800 on January 17; terms 2/10, n/30. Sales...
-
What are the factors that affect the breakeven point under? (a) Variable costing and (b) Absorption costing?
-
The president of Frogger Company is puzzled. During the last year, the company experienced a net loss of $800,000, yet its cash increased $300,000 during the same period of time. Explain to the...
-
What trend shown in Figure 15.1 helps to explain why jobs in education and protective services have the highest rates of unionization?
-
Bettner, Inc., is a calendar year corporation whose financial statements for 2012 and 2013 included errors as follows: Assume that purchases were recorded correctly and that no correcting entries...
-
1. is the business profitable? is it able to pay its current obligatiins/ liabilities? is assets are financed more by debts or equity? For the 1st quarter of 2022
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
Describe the bonding (using valence bond theory) of the Group IVA atoms in each of the following: a. C2H6 b. SiF62 c. CH3CHP==CH2 d. SiH4
-
The State of Confusion Legislature passes the following statute: "The State Health Commissioner, when in their opinion, there is sufficient covid - 1 9 vaccine that has been approved by the Federal...
-
Case 1 Baum Co. has two processing departments: Fabrication and Assembly. In the Fabrication Department, metal is cut and formed into various components, which are then transferred to Assembly. The...
-
Your earlier Personal Leadership Assessment, you looked at two areas of your leadership experience, those who led you and those you led. You will again address these two items in your Personal...
-
What is required in this situation: Content slides explaining the qualitative and quantitative steps necessary in conducting a sensitivity analysis. How can a project's risk be incorporated into a...
-
What is "marketing"? What is the difference between "marketing" and the "marketing process"?is it different in your home country vs. North america? Q2. What is the difference between "demand",...
-
What is the octane number of a mixture that is 35% heptanes and 65% is ooctane?
-
Determine by direct integration the values of x for the two volumes obtained by passing a vertical cutting plane through the given shape of Fig. 5.21. The cutting plane is parallel to the base of the...
-
A concrete form used to pour a basement wall is to hold wet concrete mix (sg = 2.6) during construction. The wall is to be 3 m high, 10 m long, and 150 mm thick. What pressure does the wet concrete...
-
An environmental instrumentation package is to be designed to be lowered into the Mariana Trench to a depth of 11 km into the Pacific Ocean. If the case is to be watertight at that depth in sea...
-
A scuba diver will descend one and a half atmospheres into a fresh water lake. Calculate the depth of the dive. Note that an atmosphere is a measure sometimes used by divers to indicate a depth in...
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


