Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 × 10-6 M magnesium chloride is added to 80.0 mL of 1.0 × 10-6 M potassium fluoride.
Appendix F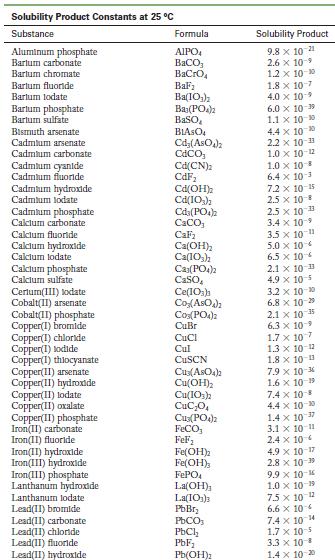

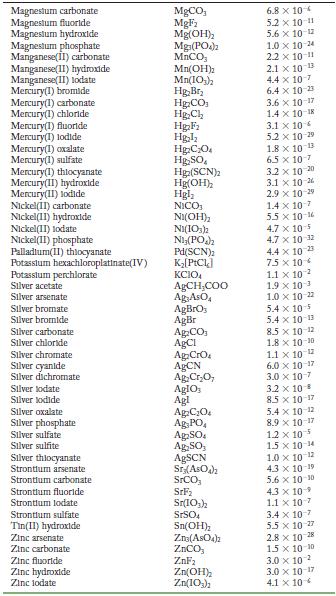
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calclum fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper(1) chloride Copper(1) todide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II)oxalate Copper(II) phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(II) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Baz(POA)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(103) Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103)1 Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cu3(ASO4)2 Cu(OH) Cu(103)2 CuC0 Cu3(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-6 1.6 x 10 19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To determine whether a precipitate will form when 200 mL of 10 106 M magnesium chloride is added to ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO 3 is added to 10 mL 0.0010 M Na 2 SO 4 . Appendix F Solubility Product...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride? Appendix F...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 10 -6 M iron(II) chloride is added to 20.0 mL of 3.0 10 -4 M barium hydroxide....
-
What is control resolution in a robot positioning system?
-
Companies that make no variable-cost/fixed-cost distinctions must use absorption costing, and those that do make variable-cost/fixed-cost distinctions must use variable costing. Do you agree? Explain.
-
The statement of cash flows has become a commonly provided financial statement by companies throughout the world. It is interesting to note, however, that its format does vary across countries. The...
-
Stock options have been called the pay program that "built Silicon Valley" because of their key role as incentive pay for employees in high-tech companies. They were popular during the 1990s, when...
-
On February 14, 2016, Isabelle Moretti, Aida Kam, and Channade Fenandoe start a partnership to operate a marketing consulting practice. They sign a partnership agreement to split profits in a 2:3:4...
-
1. Zorro Foods have provided following information about its portfolio, where the securities are classified as available for sale. Security Cost(s) Fair Value(s) Fair Value(s) 31.12.2016 31.12.2017...
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
If Cavalier Company had net income of \(\$ 672,300\) in 1997 and it experienced a \(25 \%\) increase in net income over 1996, what was its 1996 net income?
-
BREAD Products' pretax income for 2019 is * (1 Point) BREAD Products has no Work in Process or Finished Goods inventories at the close of business on December 31, 2018. The balances of BREAD's...
-
Convert the following line of code into assembly language. A (A B)+(BA) Where A and B are both 8-bit variables Activate Windows
-
14. Create a one variable Data Table from what you just copied and pasted giving the total sales for each department, and the Largest Sale from each department. Start your Criteria range in cell A1....
-
E4.1 (LO 1), C The following independent situations require professional judgment for determining when to recognize revenue from the transactions. a. Southwest Airlines sells you an advance-purchase...
-
Spring Flings Company, a fashion retailer that specializes in colorful graphic tees, prepares a master budget on a quarterly basis. The company has assembled the following data to assist in preparing...
-
Give the name or condensed structural formula, as appropriate: (c) 2,5,6-trimethylnonane (d) 3-propyl-4,5-methyldecane CH3CH2 CH2CH3 (a) CH CCH2CH CH3 CH3 CH3 (b) CH3CH2CH2CCH, CH3CHCH2CH3
-
1. What is the semi-annually compounded interest rate if $200 accumulates to $318.77 in eight years? Answer in percentage with two decimal places. 2. What is the quarterly compounded interest rate if...
-
An exhaust system for a room creates a partial vacuum in the room of 1.20 in of water relative to the atmospheric pressure outside the room. Compute the net force exerted on a 36- by 80-in door to...
-
A piece of 14-in Schedule 40 pipe is used as a pressure vessel by capping its ends. Compute the force on the caps if the pressure in the pipe is raised to 325 psig. See Appendix F for the dimensions...
-
A pressure relief valve is designed so that the gas pressure in the tank acts on a piston with a diameter of 30 mm. How much spring force must be applied to the outside of the piston to hold the...
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


