Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO3 is added to 10 mL 0.0010 M Na2SO4.
Appendix F

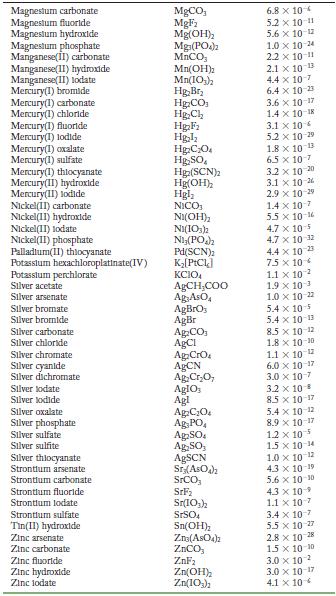
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper (1) chloride Copper (1) todide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II)oxalate Copper(II) phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(11) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Ba3(PO4)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(103) Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103)1 Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cu3(ASO4)2 Cu(OH) Cu(103)2 CuC0 Cu3(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-6 1.6 x 10-19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Q 12 10 ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride? Appendix F...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 10 -6 M iron(II) chloride is added to 20.0 mL of 3.0 10 -4 M barium hydroxide....
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 10 -6 M magnesium chloride is added to 80.0 mL of 1.0 10 -6 M potassium fluoride....
-
Write the complete APT part program to profile mill the outside edges of the part. The part is 15 mm thick. Tooling = 30 mm diameter end mill with four teeth, cutting speed = 150 mm/min, and feed =...
-
The main trouble with variable costing is that it ignores the increasing importance of fixed costs in manufacturing companies? Do you agree? Why?
-
Here are the financial statements of YoYo Company. Additional data: 1. Dividends of $36,000 were declared and paid. 2. During the year equipment was sold for $10,000 cash. This equipment cost $15,000...
-
7. Based on the balanced scorecard in Table 13.2, find the incentive pay for an employee earning a salary of$4,000 a month in each of the following situations. (LO 13-5) a. The company met all of its...
-
On November 1, 2018, Sigma Inc. invests in $1,000,000 of 5%, 10-year bonds issued by Microsoft Corporation, intending to hold the bonds until their maturity. The bonds pay interest each January 1 and...
-
Perry Co set up a petty cash fund for payments of small amounts. The following transactions involving the petty cash fund occurred in . Ray 1 Prepared a company check for $400 to establish the petty...
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
Ron Howard recently took over as the controller of Johnson Brothers Manufacturing. Last month, the previous controller left the company with little notice and left the accounting records in disarray....
-
Why the sudden increase in income before taxes in 2021? 8. Why were the operating assets the highest in 2019? 9. Why are the short-term loans the highest in 2020? 10. Why are the other long-term...
-
Mercy wants to make sure that she will be able to provide for her daughter's college and plans to open a savings account with a bank that is ready to pay interest as shown below per year compounded...
-
Question 1. For a firm that uses portfolio management, please give a real or hypothetical example of how the CEO's personal bases for power help organizational performance. Question 2. Give a real...
-
Make a schedule that you would use that effectively illustrates working with paraprofessionals that includes collaboration time. Use the examples provided in the following resources to guide your...
-
How does the integration of technology and automation influence employee motivation and job satisfaction within modern organizational contexts ?
-
Give the name or condensed structural formula, as appropriate: (b) 2,2-dimethylpentane (c) 4-ethyl-1,1-dimethylcyclohexane (d) (CH3)2CHCH2CH2C(CH3)3 (e) CH3CH2CH(C2H5)CH2CH2CH2CH3 (a) CH3CHCH3...
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
If air has a constant specific weight of 0.075 lb/ft 3 , what pressure difference would result when driving from the base to the top of Pikes Peak, if the climb for the trip is 8400 ft?
-
Figure 4.21 shows a vacuum tank with a flat circular observation window in one end. If the pressure in the tank is 0.12 psia when the barometer reads 30.5 in of mercury, calculate the total force on...
-
The flat left end of the tank shown in Fig. 4.21 is secured with a bolted flange. If the inside diameter of the tank is 30 in and the internal pressure is raised to +14.4 psig, calculate the total...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...

Study smarter with the SolutionInn App


