Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 × 10-6 M iron(II) chloride is added to 20.0 mL of 3.0 × 10-4 M barium hydroxide.
Appendix F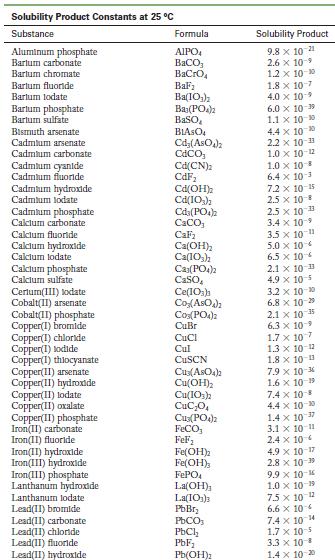

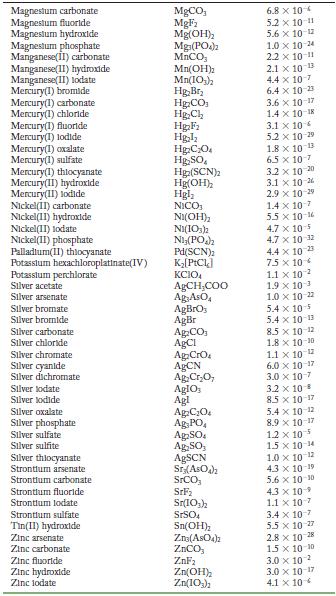
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calcium fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper(1) chloride Copper(1) lodide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II) oxalate Copper(II)phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(11) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Baz(PO4)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(10) Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103)1 Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cuz(ASO4)2 Cu(OH) Cu(10)2 CuC0 Cu3(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-6 1.6 x 10 19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Q 53 10 ...View the full answer

Answered By

Amit Singh
When I was preparing for IIT-JEE exam which is one of the toughest exam of India , I use to teach mathematics and physical chemistry to my friend.
During college days I use to teach structural analysis, strength of materials,RCC i.e. Civil Engineering topics to my friends.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO 3 is added to 10 mL 0.0010 M Na 2 SO 4 . Appendix F Solubility Product...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 10 -6 M magnesium chloride is added to 80.0 mL of 1.0 10 -6 M potassium fluoride....
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride? Appendix F...
-
How are Bit coins different from VCU1, VCU2, and VCU3 currencies? How are they similar? What is the primary economic threat of Bit coins?
-
Give an example of how, under absorption costing, operating income could fall even though the unit sales level rises.
-
An analysis of comparative balance sheets, the current years income statement, and the general ledger accounts of Coffee Table Corp. uncovered the following items. Assume all items involve cash...
-
Describe new approaches to labor management relations.
-
Relating market value to book value of shareholders' equity Firms prepare their balance sheets using authoritative guidance for the recognition and measurement of assets and liabilities. Accountants...
-
Newland Company reported retained earnings at December 31, 2021, of $330,000. Newland had 200,000 shares of common stock outstanding at the beginning of 2022. The following transactions occurred...
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
An employee can implement a kickback scheme regardless of whether she has approval authority over the purchasing function. How might this be accomplished?
-
What are knowledge or innovation workers? What are the key elements of professional practice, work environment and work design needed to support the productivity and creativity of knowledge or...
-
What problem does this concept solve or what pain does it alleviate and how compelling is the problem? 2. Who is your specific target customer? 3. How do they currently meet this need for themselves...
-
Consider a person standing in a room where the average wall temperature is 20 C. This person is trying to reach the "thermal comfort" by adjusting the A/C air temperature. Find out the appropriate...
-
If WHO, the World Health Organization,defines health as a state of completephysical, mental and social well-being and not merely the absenceof disease and infirmity (WHO, 2011)and wellness is...
-
As a manager, you want to find a way to motivate Nate and increase his engagement and job satisfaction in the workplace. Drawing upon a behavioral theory of motivation, discuss how you, as a manager,...
-
Give the name or condensed structural formula, as appropriate: (a) 3-phenylpentane (b) 2,3-dimethylhexane (c) 2-ethyl-2-methylhepane (d) CH3CH2CH(CH3)CH2CH(CH3)2 ,
-
In Problem 8.43, determine the smallest value of for which the rod will not fall out of the pipe. IA -3 in.-
-
An inclined manometer similar to the one shown in Figure 3.14 is used for sensitive pressure measurement. It is inclined at an angle of 25 degrees above the horizontal and uses red gage fluid with a...
-
A meteorologist reports a high pressure system with barometric pressure of 790 mm of mercury and then later in the year a low pressure system with a pressure of 738 mm of mercury. What is the total...
-
What is the pressure, in psig, at the bottom of a swimming pool that is 10 ft deep?
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


