What is H at 25 C and 1.00 atm for the combustion of 1 mol ethane if
Question:
What is ΔH at 25 °C and 1.00 atm for the combustion of 1 mol ethane if ΔE = -1553 kJ?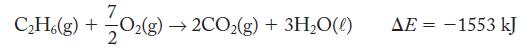
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
AH...View the full answer

Answered By

AJIN KURIAKOSE
I HAVE ELECTRONICS ENGINEERING DEGREE..AND MY AREA OF INTEREST IS MATHEMATICS,CONTROL SYSTEM,NETWORK,DIGITAL
4.70+
21+ Reviews
32+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
How much heat, in kilojoules, is evolved in the complete combustion of (a) 1.325 g C 4 H 10 (g) at 25 C and 1 atm; (b) 28.4 L C 4 H 10 (g) at STP; (c) 12.6 L C 4 H 10 (g) at 23.6 C and 738 mmHg?...
-
The difference in the standard free energies of formation for 1-butene and 2-methylpropene is 13.4 kJ mol 1 (3.2 kcal mol 1 ). (See the previous problem for a definition of G f .) (a) Which compound...
-
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO 2 they produce. The greater the heat relative to the...
-
The Sellinger Business School's Information Technology Service (ITS) is considering a new process to refurbish older computers in order to save on costs of buying new computers. The five steps to the...
-
One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehydes 3-phosphate. The process occurs by phosphorylation with...
-
In Problem S7-19, suppose the division commanders are limited to three possible sites for the supply depot because of airfield locations and enemy troop concentrations. The coordinates (in miles) of...
-
Have production-operations goals been established, and are work activities aimed at achieving these goals?
-
On January 1, 2010, the Field Company acquired 40% of the North Company by purchasing 8,000 shares for $144,000 and obtained significant influence. On the date of acquisition, Field calculated that...
-
explain the impact of foreign exchange risk, economis risk, sovereign risk and political risk on the credit analysis of international loans.
-
Determine the entropy change when 1 mol H 2 O freezes at its normal freezing point of 0.0 C. Th e heat of fusion of water is 6.01 kJ/mol.
-
A 7.56-g sample of gas is in a balloon that has a volume of 10.5 L. Under an external pressure of 1.05 atm, the balloon expands to a volume of 15.00 L. Then the gas is heated from 0.0 C to 25.0 C. If...
-
(a) If $4000 is invested at 1.75% interest, find the value of the investment at the end of 5 years if the interest is compounded (i) Annually, (ii) Semiannually, (iii) Monthly, (iv) Weekly, (v)...
-
You are required to work with your groups for the restaurant business that you have created and develop your international market entry strategy. Please follow the below steps: STEP 1: Research the...
-
Demonstrate to the owner of the business how they could use e-commerce for example (Shopify, E-Bay, Etsy), social media for example (Facebook, Instagram, TikTok, Webpage) to market their business to...
-
Identify a company that has successfully created a brand image for their product or service by using social media. Explain how they did it . Which forms of media did they use?
-
The Magic that Makes Customer Experiences Stick Article Identify and explain in details Roger's Five Factors as it applies to the diffusion process.
-
Topic: Buick in China Task: After reading and viewing the items in this week's Reading & Study folder, identify and describe the following: the social and cultural aspects that made China attractive...
-
The visible emission lines observed by Balmer all involved nf = 2. (a) Explain why only the lines with nf = 2were observed in the visible region of the electromagnetic spectrum. (b) Calculate the...
-
3M Company reports the following financial statement amounts in its 10-K report: a. Compute the receivables, inventory, and PPE turnover ratios for both 2018 and 2017. (Receivables turnover and...
-
A horse galloped a mile in 2 min 35 s. What was its average speed in km/h?
-
Figure P.4.95 is a plot of n I and n R versus λ for a common metal. Identify the metal by comparing its characteristics with those considered in the chapter and discuss its optical...
-
Convert 0.296 cm 3 /s to m 3 /s.
-
TB SA Qu. 13-74 (Static) What must Abdu invest today to... What must Abdu invest today to receive an annuity of $9,000 for four years semiannually at an 8% annual rate? All withdrawals will be made...
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...

Study smarter with the SolutionInn App


