Write the rate law and the molecularity for each of the following elementary reactions. (a) CH5C1 CH4
Question:
Write the rate law and the molecularity for each of the following elementary reactions.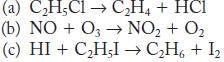
Transcribed Image Text:
(a) CH5C1 CH4 + HC1 (b) NO + O3 NO + O (c) HI + CHI CH6 + 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a rate k CH5Cl u...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the expected rate law and molecularity for each of the following elementary reactions in the gas phase. Strategy The rate law for an elementary reaction is derived directly from its...
-
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) 2 NO(g) N2O2(g) (c) SO3(g) SO2(g) + O(g)? CH2 H2C-CH2(g)- CH2_ CH-CH3(g)
-
Write the rate law and the molecularity for each of the following elementary reactions. (a) NO+NOCl NO + NOCI (b) NO + SO NO + SO3 (c) NO4 2NO
-
The following list of balances has been extracted from the records of company cowgale co as of 31 October 2017 the end of the most recent financial year. Notes 1. The balance on the corporation tax...
-
Find Vae and Vcf in the circuit infigure. a 9V 5V 12 V
-
Ren Levin wishes to determine the future value at the end of 2 years of a $15,000 deposit made today into an account paying a nominal annual rate of 12%. a. Find the future value of Rens deposit,...
-
How ethical is it for Southwest managers and HR specialists to make observations about the behavior of job candidates even when the candidates do not know that they are being evaluated? LO.1
-
(a) Determine the amount of work (in joules) that must be done on a 100-kg payload to elevate it to a height of 1 000 km above the Earths surface. (b) Determine the amount of additional work that is...
-
On the first day of the fiscal year, a company issues a $2,000,000, 8%, 4-year bond that pays semiannual interest of $80,000 ($2,000,000 8% ), receiving cash of $2,215,104. Journalize the bond...
-
Nitrogen dioxide reacts with carbon monoxide to form carbon dioxide and nitrogen monoxide. NO(g) + CO(g) CO(g) + NO(g) Two mechanisms are proposed: Mechanism I (one step): NO(g) + CO(g) CO(g) + NO(g)...
-
Evaluate each of the following proposed mechanisms to determine whether it is consistent with the experimentally determined stochiometry and rate law, and identify intermediates, if any. 2NO2+O3 NO5...
-
The concentration of lactate in blood rises sharply during a sprint and declines slowly for about an hour afterward. What causes the rapid rise in lactate concentration? What causes the decline in...
-
Bybee Printing makes custom posters and is currently considering making large-scale outdoor banners as well. Which one of the following is the best example of an incremental operating cash flow...
-
https://filmsfortheearth.org/en/film/humans-destroyers-of-earth/ This documentary will give you more insight into the speed at which globalization has taken place during the dawn of...
-
1. Write and explain (through comment or description after the program) a complete C program to perform the following activities: a. Take Student ID as user input from keyboard (2) b. Determine the...
-
Assume that I have a Company uses a job costing system with machine hours as the allocation base for overhead. The company uses normal costing to develop the overhead allocation rate. The following...
-
(CASE STUDY )Company Information: ABC Company is a large automotive dealer company operating in the field of automobile retailing that is owned by a big Holding Group Company XYZ. ABC Company buys...
-
Predict the products of the following reactions. (a) (b) HCI DCI (D-2H)
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Determine the vertical displacement of joint D. Using Castiglianos theorem. AE is constant. Assume the members are pin connected at their ends. 500 lb 300 lb -3 ft -3 ft- 3 ft 600 lb
-
Determine the vertical displacement of joint D. Use the method of virtual work. AE is constant. Assume the members are pin connected at their ends. 500 lb 300 lb -3 ft -3 ft- 3 ft 600 lb
-
Determine the vertical displacement of joint D. Using Castiglianos theorem. AE is constant. Assume the members are pin connected at their ends. 000 4 m 4 m - 15 kN 20 kN 000
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


