In Problem convert the given i-system to an e-system using slack variables. Then construct a table of
Question:
In Problem convert the given i-system to an e-system using slack variables. Then construct a table of all basic solutions of the e-system. For each basic solution, indicate whether or not it is feasible.
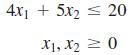
Transcribed Image Text:
4x1 + 5x2 < 20 X1, X2 2 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

College Mathematics For Business Economics, Life Sciences, And Social Sciences
ISBN: 978-0134674148
14th Edition
Authors: Raymond Barnett, Michael Ziegler, Karl Byleen, Christopher Stocker
Question Posted:
Students also viewed these Mathematics questions
-
For each item listed, state whether or not it would be disclosed for each of the reportable segments, identify the segments for which it would be disclosed and explain what other disclosures, if any,...
-
Each day, a weather forecaster predicts whether or not it will rain. For 80% of rainy days, she correctly predicts that it will rain. For 90% of non-rainy days, she correctly predicts that it will...
-
For each of the following scenarios, indicate whether or not independence related SEC rules are being violated, assuming that the audit entity is a public company. Briefly explain why or why not. a....
-
Problem 4: The two compensator circuits (Fig. P4a) are used to improve the closed-loop system performance: 1) Derive the transfer functions to characterize the two circuits, and show that they can be...
-
What is the interpretation of income tax expenses under partial allocation?
-
Ellen Bright, CEO of TWA Parts (TWAP), looked at the two balanced scorecards before her. One, from the luxury division, showed strong financial results; while the other, from the economy division,...
-
Oneida Associates is a real estate company operating in the Finger Lakes region of central New York. Its leasing division rents and manages properties for others, and its maintenance division...
-
Assume that Atlas Sporting Goods Inc. has $840,000 in assets. If it goes with a low-liquidity plan for the assets, it can earn a return of 15 percent, but with a high-liquidity plan the return will...
-
Notes Payable 6,000 Unearned Service Revenue 5.920 1.432 10.320 3,820 61.820 11.620 Salaries and Wages Payable Common Stock Retained Earnings Service Revenue Salaries and Wages Expense Insurance...
-
Adam and Poh, two young entrepreneurs, wished to form a small proprietary limited company to operate a restaurant. The company was to be called 'Master Plate Pty Ltd' On 22 February, Adam entered...
-
In Problem (A) Form the dual problem. (B) Is the dual problem a standard maximization problem in standard form? Explain. Minimize C = 4x1 x2 subject to 5x1 + 2x, 2 7 4x, + 6x, 2 10 X1, X2 2 0
-
A refinery produces two grades of gasoline, regular and premium, by blending together three components: A, B, and C. Component A has an octane rating of 90 and costs $28 a barrel, component B has an...
-
The Insurance Institute for Highway Safety (IIHS) routinely conducts crash tests on vehicles to determine the cost of repairs. The following data represent the vehicle repair costs for 2009-model...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Barb Company has provided information on intangible assets as follows: 1. A patent was purchased from Lou Company for $1,500,000 on January 1, 2018. Barb estimated the remaining useful life of the...
-
3M Company reports the following financial statement amounts in its 10-K report: a. Compute the receivables, inventory, and PPE turnover ratios for both 2018 and 2017. (Receivables turnover and...
-
A marine manufacturer will sell N(x) power boats after spending $x thousand on advertising, as given by (see figure). (A) Find N(x). (B) Find N(10) and N(20). Write a brief verbal interpretation of...
-
The ozone level (in parts per billion) on a summer day in a metropolitan area is given by P(t) = 80 + 12t - t 2 where t is time in hours and t = 0 corresponds to 9 A.M. (A) Use the four-step process...
-
Let f be defined by S1 + mx if xs1 14 - mx if x > 1 f(x) where m is a constant. (A) Graph f for m = 1, and find lim f(x) and lim f(x) (B) Graph f for m 2, and find lim f(x) lim f(x) and (C) Find m so...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


