If the PCI editor at Chemical Engineering (CE) decides to update the Plant Cost Index so that
Question:
Table 15-3
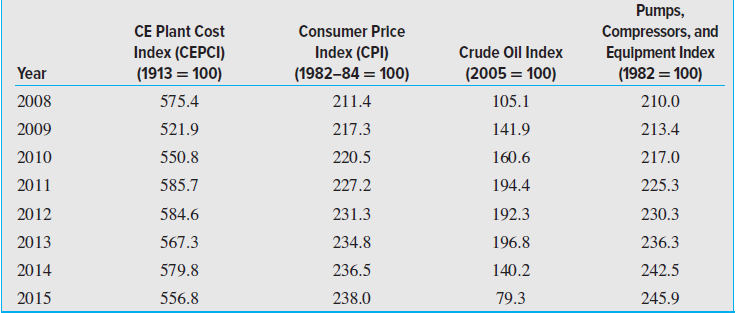
Transcribed Image Text:
Pumps, Compressors, and Equipment Index (1982 = 100) CE Plant Cost Consumer Price Index (CPI) (1982-84 = 100) Index (CEPCI) Crude Oil Index (1913 = 100) Year (2005 = 100) 575.4 105.1 2008 211.4 210.0 2009 521.9 217.3 141.9 213.4 2010 220.5 550.8 160.6 217.0 2011 194.4 585.7 227.2 225.3 2012 231.3 584.6 192.3 230.3 2013 567.3 234.8 196.8 236.3 236.5 2014 579.8 140.2 242.5 2015 556.8 238.0 79.3 245.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
PCI for 2010 55085508 100 PCI for 2014 57985508 1...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Business questions
-
Jennifer Ozman is a sales engineer at Montana Chemical Engineering Company. Jennifer owns two vehicles, and one of them is entirely dedicated to business use. Her business car is a used, small pickup...
-
If the editors at ENR decide to redo the construction cost index so that the year 2000 has a base value of 100, determine the value for the year (a) 1995 (b) 2009.
-
Pinnacle Engineering, an acclaimed chemical engineering team of engineers, chemists, and other scientists, had the following income in 2017: The following changes are expected in 2018: 1. The company...
-
Data Set 32 "Airport Data Speeds" in Appendix B includes Sprint data speeds (mbps). The accompanying TI-83 / 84 Plus display results from using those data to test the claim that they are from a...
-
Wellington International Airport Limited is a for-profit company domiciled in New Zealand that manages the Wellington Airport and files its financial statements in compliance with IFRS. For the...
-
A sample of 36 observations is selected from a normal population. The sample mean is 49, and the population standard deviation is 5. Conduct the following test of hypothesis using the .05...
-
4. What is the nature of the nonrecurring loss appearing on the consolidated income statement? Reproduce the consolidating entry from which this figure originated and explain. 5. What is the amount...
-
J& B Drilling Company has recently acquired a lease to drill for natural gas in a remote region of southwest Louisiana and southeast area has long been known for oil and gas production, and the...
-
help asap!!! e tolowng accounts, with the balances ind caled, appear in the ledger of Garcon Co on December 1 of the current year: The folowing transactions felating to peypot, payrol dedvctions, and...
-
1. If you were Kelly, what would you tell the CFO? 2. Suppose the union insists on including one of the following stipulations in the agreement: a. No more than half of the total number of bonds...
-
Why do many entrepreneurs underprice their goods and services, especially when they first get into business? Discuss the connection between the prices a company establishes for its goods and services...
-
Work with a team of your classmates to define the ethical issues involved in dynamic pricing.
-
At a federal trial, expert testimony concerning Mayan hieroglyphics is required. One party offers the testimony of Roberto Rinaldo, who speaks several ancient Mayan dialects and was the first to...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Flin Flon Company enters into a contract on April 3, 2024, with Thompson Industries to supply 5,000 microprocessors at a price of $7 each, terms n/30. The microprocessors cost Flin Flon $3 each. The...
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
Explain how a compensation plan could be developed to provide incentives for experienced salespeople and yet make some provision for trainees who have not yet learned the job.
-
Are the benefits and limitations of a canned presentation any different if it is supported with a Power- Point presentation or DVD than if it is just a person talking? Why or why not?
-
How would our economy operate if personal salespeople were outlawed? Could the economy work? If so, how? If not, what is the minimum personal selling effort necessary? Could this minimum personal...
-
Draw an interaction diagram for the following contract: If a collaboration diagram is used, be sure that all messages' sequence numbers are shown. Contract: Scheduling a Flight Operation:...
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:

Study smarter with the SolutionInn App


