(a) Use data from Appendix 11 to construct a potential diagram showing the redox chemistry of vanadium...
Question:
(a) Use data from Appendix 11 to construct a potential diagram showing the redox chemistry of vanadium in aqueous solution at pH0.
(b) Use your diagram to establish whether any vanadium species is unstable with respect to disproportionation.
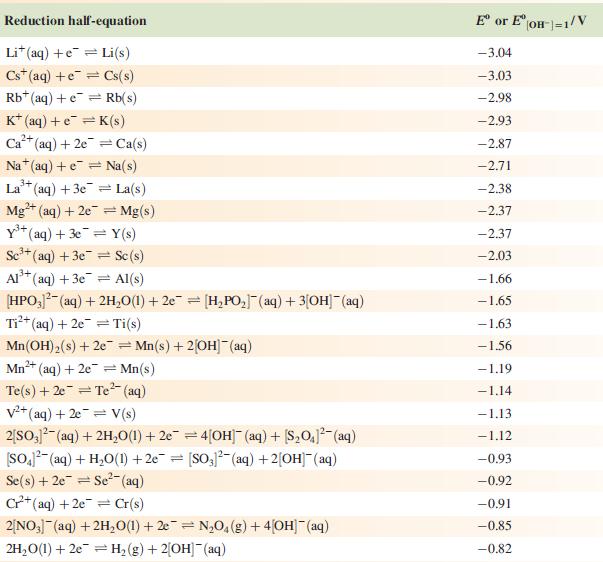
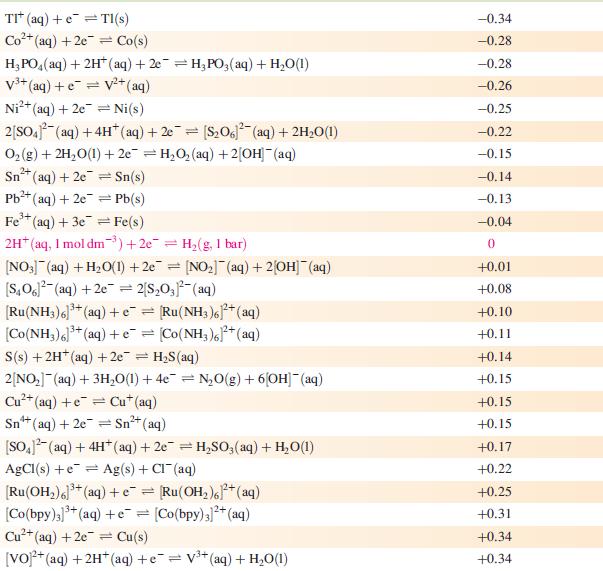
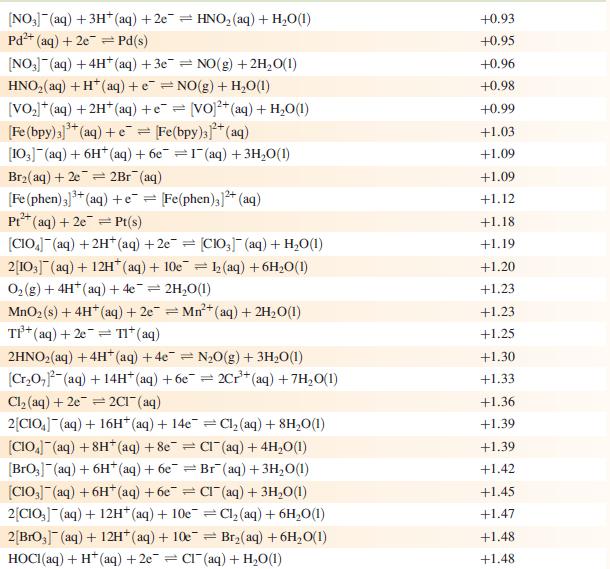
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1

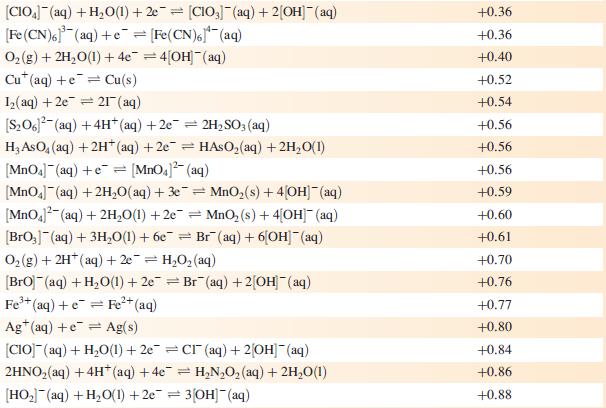

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





