The compound H 3 N BH 3 is an adduct of NH 3 and BH 3
Question:
The compound H3N · BH3 is an adduct of NH3 and BH3. It is currently being investigated as a possible hydrogen storage material.
(a) What is the hydrogen storage capacity (percentage by weight) of H3N · BH3?
(b) Using the VSEPR model, draw structures for BH3, NH3 and H3N · BH3.
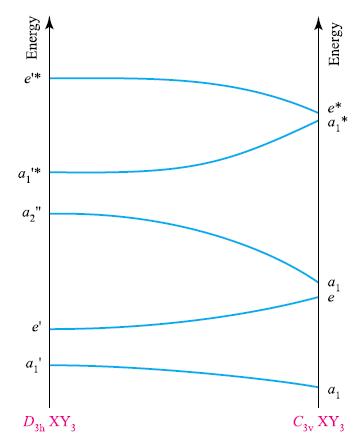
(c) Figure 5.36 shows how the orbital energies of a D3hXY3 molecule alter as the molecular geometry changes. This type of diagram is called a Walsh diagram. Why are the orbital symmetry labels different at the two sides of the diagram?
(d) Which of the orbitals in Fig. 5.36 represent the HOMO and LUMO of BH3 and NH3?
(e) Sketch representations of the MOs to which the orbital symmetry labels in Fig. 5.36 refer.
(f) Suggest how BH3 and NH3 interact to form H3N · BH3. What type of N–B bond is present?
Figure 5.36
Step by Step Answer:






